
UPDATE: SPIKE PROTEIN ENDOTHELIAL DISEASE (SPED): THE INVOLVEMENT OF THE MITOCHONDRIA: WHY METFORMIN WORKS: WHY SUGAR IS DELETERIOUS
The Mitochondria are involved in the formation of new vessels… and the regression of preexisting ones
Significant players in SPED are, I believe, the Mitochondria. Why I find this fascinating are the circumstances of apoptosis in endothelial cells.
During both normal development and pathology, the formation of new vessels and the regression of preexisting ones are dependent on the balance between endothelial cell proliferation and endothelial cell apoptosis.
Apoptosis in the vasculature: mechanisms and functional importance
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1572165/
The Mitochondria are the orchestrators of Apoptosis in the Endothelium.
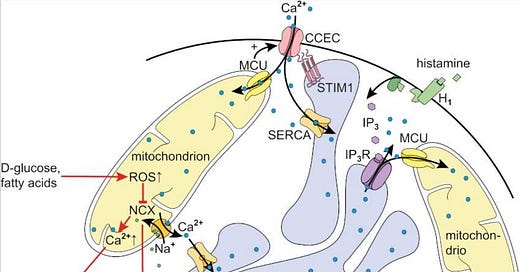
Endothelial mitochondria are essential to the functional integrity of the endothelial cell as they integrate a wide range of cellular processes including Ca2+ handling, redox signaling and apoptosis, all of which are closely interrelated.
Endothelial mitochondria—less respiration, more integration
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3387498/
Given the Spike Protein’s interaction with the Endothelium, especially via the disruption of Calcium homeostasis, triggers Endothelial cell Apoptosis. The Spike Protein alone causes an influx of extracellular Ca2+.
We found that an upstream calcium-dependent PKC isozyme PKC alpha that modulates the downstream ERK/NF-kappaB pathway through an influx of extracellular Ca2+ is induced by the spike protein of SARS-CoV.
Spike protein of SARS-CoV stimulates cyclooxygenase-2 expression via both calcium-dependent and calcium-independent protein kinase C pathways
https://www.researchgate.net/publication/6537904_Spike_protein_of_SARS-CoV_stimulates_cyclooxygenase-2_expression_via_both_calcium-dependent_and_calcium-independent_protein_kinase_C_pathways
Excess Mitochondrial Ca2+ can trigger Apoptotic pathways.
In endothelial cells, inhibition of NCX by either pharmacological compounds [91] or treatment with ROS causes prolonged mitochondrial Ca2+ elevation and, in consequence, also insufficient Ca2+ refilling of the ER during continuous stimulation, the latter of which has been generally neglected so far. This impairment of cellular Ca2+ signaling could conceivably affect endothelial function and, in the worst case, both excess mitochondrial Ca2+ and ER stress could trigger apoptotic pathways.
It is through this Apoptotic mechanism that MICROANGIOPATHY is induced, leading to the destruction of the microvasculature.
Mitochondria have a critical function in triggering this enzymatic cascade of self-destruction by releasing a set of proteins into the cytosol. This happens in response to proapoptotic stimuli that can originate either from inside the cell itself or from outside by activation of death receptors. A lot of in vitro studies suggest that endothelial cell apoptosis might play a special role in the pathophysiology of micro- and macroangiopathy under certain conditions like diabetes or hyperlipidemia.
It is through a counterintuitive mechanism that the excess calcium in the cytosol causes the calcium WITHIN the Mitochondria to build up, opening a pore (called mPTP) in its outer membrane, allowing for the Apoptotic signaling to commence.
Mitochondrial Ca2+ overload is one of the factors that independently cause opening of mPTP. During sustained elevations of cytosolic Ca2+, observed in multiple pathological conditions of the endothelium, free Ca2+ inside the mitochondrial matrix progressively rises. In the presence of cyclophilin D, this induces the formation of mPTP that is kept open as long as the cytosolic Ca2+ elevation persists. The pore allows solutes to freely diffuse into the mitochondrial matrix leading to mitochondrial swelling and rupture of the OMM followed by release of proapoptotic factors.
ENTER METFORMIN
Metformin has proven to be an exceptional therapeutic against COVID, and, I believe, will be against the Spike Protein. Why? A miraculous ability which the drug has.
From a therapeutic point of view, the antidiabetic agent metformin is of special interest because in addition to its metabolic effects, it prevents the opening of mPTP.
Preventing the opening of mPTP will prevent the Apoptotic death of the Endothelial cell, thereby preventing the induction of the cascade of damage/healing leading to microvascular destruction.
THIS EXPLAINS WHY DIABETES AND HIGH BLOOD SUGAR ARE MAJOR RISK FACTORS
High d-glucose also provokes alterations in mitochondrial dynamics and Ca2+ signaling that might even precede the ROS burst itself and, thus, represent attractive targets for future therapeutic interventions.
Endothelial mitochondria—less respiration, more integration
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3387498/
These findings, I believe, further elucidate the dangers of the Spike Protein towards the Microvasculature, yet show us ways to hopefully prevent further damage and heal ourselves.
Your support and appreciation keep me going. Let’s continue to discover and find answers.












Much appreciated, Walter! Your identification of the role of the mitochondrial permeability transition pore (mPTP) and the ability of metformin to help it stay closed to protect the mitochondria should enable those who learn of it help themselves and their patients reduce the damage caused by these (apparent bioweapon) spike proteins. I will do what I can to spread the word.
Metformin natural alternative: B12. Also used to treat radiation poisoning. Hmmmm. https://www.webmd.com/vitamins/condition-1863/metformin-related-vitamin-b12-deficiency