
Friday Hope: ß-Hydroxybutyrate (BHB): Amyloid Inhibition and Improved Mitochondrial Function
ß-Hydroxybutyrate may be the reason intermittent fasting is beneficial for those with COVID/Long COVID/Spike related pathologies.
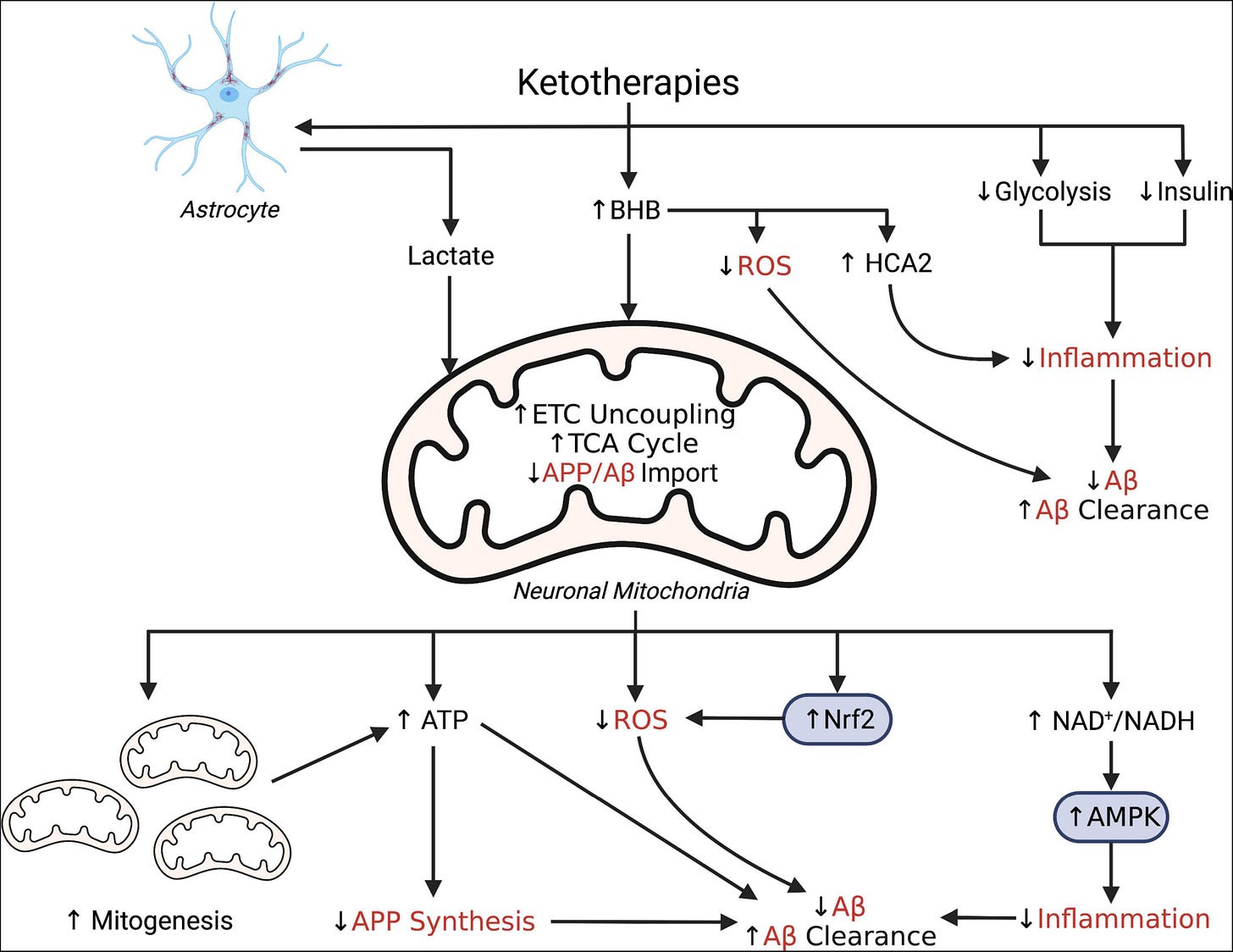
Simplified schematic illustrating putative pleiotropic effects of ketotherapies as modulators of amyloid-β. Ketotherapies (KTs) potentially modulate amyloid-β (Aβ) through various direct and indirect mechanisms targeting poor mitochondrial bioenergetics, increased ROS, and increased inflammation. KTs, especially the ketogenic diet (KD), reduce systemic insulin and potentially improve peripheral metabolic status which may improve systemic inflammation and reduce Aβ. The ketone body, β-hydroxybutyrate (BHB), serves as an energy substrate for mitochondrial metabolism, upregulates the astrocyte-neuron lactate shuttle, activates hydrocarboxylic acid receptor 2 (HCA2) to regulate inflammation, and may directly scavenge reactive oxygen species (ROS). Through bioenergetic effects in the mitochondria, KTs stimulate genesis of new mitochondria, increase uncoupling of the electron transport chain (ETC) to increase ATP production, generate less ROS than glucose metabolism, and reduce mitochondrial import of amyloid precursor protein (APP) and Aβ. KTs also activate nuclear factor-E2 related factor 2 (Nrf2) to upregulate synthesis of ROS-scavenging antioxidants and AMP-activated protein kinase (AMPK) to regulate transcription of pro-inflammatory cytokines.
Potential for Ketotherapies as Amyloid-Regulating Treatment in Individuals at Risk for Alzheimer’s Disease
https://www.frontiersin.org/articles/10.3389/fnins.2022.899612/full
As evidence continues to emerge that the Spike Protein is not only amyloidogenic but also disrupts mitochondrial function, there is a need to find therapeutics which address these pathologies. It has also been shown that those on a low carb diet tend to fare better when faced with SARS-CoV-2 and its Spike Protein.
Recent findings underlined immune advantages derived from ketone bodies, such as blockage NLRP3 inflammasome, reduction in chronic activation of ILCs and induction of protective γδ T-cells against infections. Taken together, in addition to the benefit of airway inflammation prevention by impairing the formation of lipid droplets, KDs could be an excellent tool to prevent the infection and stem the damage induced by COVID-19 in the fragile population affected from obesity.
Studies conducted in mice highlighted the beneficial effect of HFD- induced ketone bodies in COVID-19 models. In humans, HFKDs has been experimented in Intensive Care Units (ICU) and good results have been reported in mechanically ventilated patients. Moreover, telemedicine achieved good results in pediatric epileptic patients under HFKDs, either for safety and compliance, proving that it can be a valid tool to be adopted even in the event of quarantine and fiduciary isolation. On the basis of these considerations, several authors proposed KDs in COVID-19 management and some clinical trials are ongoing.
Ketogenic Diet as a Preventive and Supportive Care for COVID-19 Patients
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8003632/
As we shall see, the ability of ß-Hydroxybutyrate to inhibit the NLRP3 inflammasome may be one of the two reason why ß-Hydroxybutyrate alone could be the reason for the success of intermittent fasting in relation to COVID and Spike Protein disease.
The NLRP3 inflammasome is critical in the deposition of amyloid plaques. BHB’s inhibition of the NLRP3 inflammasome may prevent the deposition of amyloids created by the Spike Protein.
Inhibition of the NLRP3 inflammasome could also prevent cognitive decline and dementia. β-amyloid protein, which aggregates into the amyloid plaques characteristic of Alzheimer’s disease, activates the NLRP3 inflammasome in microglia, the resident macrophage population in the brain, releasing inflammatory cytokines including IL-1β. This activation is evident in the brain of humans with both mild cognitive impairment and Alzheimer’s disease, and Alzheimer’s mouse models that carry deficiencies in NLRP3 inflammasome components are protected from β-amyloid deposition and cognitive decline.
β-Hydroxybutyrate: A Signaling Metabolite
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6640868/
Now we come to the second major reason why BHB may be a “silver bullet” in treating COVID/Spike disease. BHB improves mitochondrial function.
One of the major problems the Spike Protein causes is disrupting the mitochondria. It is interesting that in the first 24 hours of Spike exposure, the mitochondria INCREASE their energy production. However, this effect is short lived and, ultimately, the Spike greatly decreases the ability of the mitochondria to produce energy. Think of it as a dimmer switch on the mitochondria.
To the best of our knowledge, the present study is the first to report that a short-term (24 h) treatment with S1 promotes ATP synthesis, increases basal OCR capacity, and preserves OCR capacity in AC16 cells. These adaptive responses help cells tolerate the S1-induced stress. Many viruses use the host cellular metabolic machinery to synthesize the energy required for viral replication by modulating ion channels; viral proteins may form a hydrophobic pore to facilitate ion transport across the cell membrane. Thus, the short-term effect of S1 on ATP synthesis may contribute to happy hypoxemia in COVID-19, in which patients exhibit severe hypoxemia without any sign of respiratory distress. However, these effects were not maintained after 48 or 72 h. By contrast, substantial decreases were noted in ATP synthesis; basal, maximal, and spare OCR capacities; and nonmitochondrial respiration. The 24 h treatment increased proton leakage, which might have led to the accumulation of ROS and impaired mitochondrial function during prolonged treatment (72 h). The energy required for the contraction of cardiomyocytes is generated primarily through mitochondrial oxidative phosphorylation. Thus, decreased mitochondrial ATP synthesis impairs cardiac function. Using human brain microvascular endothelial cells, a study revealed that the S1 decreased mitochondrial respiration.
Spike Protein Impairs Mitochondrial Function in Human Cardiomyocytes: Mechanisms Underlying Cardiac Injury in COVID-19
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10046940/
Here, again, BHB can act as a cavalry swooping in to aid the mitochondria.
The bulk of research exploring the mitochondrial effects of ketones use neurons as the cell of interest, which is not surprising given the pronounced neurological benefits of ketones, including reduced seizures, migraines and improved cognition in both states of compromised and healthy neurological function. Some of these reported mitochondrial benefits of ketones in neurons are a reduction in oxidative stress and enhanced survival, similar to what we found in muscle cells in the studies herein. Despite this neuron-heavy focus, some previous evidence exists that suggests a mitochondrial benefit in muscle, albeit indirectly. Ahola-Erkkila et al., in studying a model of mitochondrial myopathy, found that β-HB helped maintain mitochondrial respiration and morphology within muscle tissue, potentially slowing muscle loss with myopathies. This, combined with our observation of enhanced β-HB-induced myotube viability, presents a provocative paradigm with states of ketosis—that ketones promote muscle cell survival. Thus, perhaps ketones, in addition to serving other roles, act to preserve muscle mass in insulin-reduced or -deficient states, such as untreated type 1 diabetes, fasting, or a ketogenic diet. Beyond skeletal muscle, it is tempting to speculate that ketones may also alter cardiac muscle viability. Aubert et al. recently found that heart failure due to hypertrophic cardiomyopathy is typified by a fuel shift, wherein cardiomyocytes rely more heavily on ketone catabolism. Moreover, Sato et al. observed an increased ATP efficiency in myocardium fueled with ketones compared with other fuel sources.
β-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6121962/
The beneficial effects of BHB appear to be very complex in nature. Not a direct therapeutic against the Spike or SARS-CoV-2, but one that happens to possess just the right capabilities to set the stage for success. A character actor without which the plot simply could not be.
The success of intermittent fasting and low carb dieting with COVID and Spike-related disease may be in a great part due to the induction of the production of BHB. More testing and studies need to be performed. Yet, given the results so far, BHB may be our BFF.
I wish all a wonderful, joyous and Happy Thanksgiving! As always, thank you for your support and readership!











Since spring of 2022 I have been doing one 36-hr fast per week (typical Mondays are my day to not eat) because the shedding of vaxxed people affects me in an autoimmune kind of way (I have a latent connective tissue disease). I am not jabbed, but many in my family and friends are and being in the car for long rides, etc., takes its toll on me. So far, I am very happy with how I am feeling with the extended fasting. (I'm 63 and on no meds whatsoever)
Every study I see confirms the crazy meat diet folks; go low carb / keto carnivore, making beef central to the diet and enjoy your life.
It is almost as if we are supposed to just eat ruminant meat, like our ancestors did for a million of so years, everything else being candy or starvation food.