THE PURPOSE OF THE FURIN CLEAVAGE SITE AND THE GP120 INSERT IS TO INDUCE SYSTEMIC FIBROSIS AND CANCER VIA THE ENDOTHELIUM: SPED GP120 AND FURIN. THE FURIN FEEDBACK LOOP. FIBROSIS AND ONCOGENESIS.
FURIN expression as the unifying determinant of COVID-19 and Spike Protein pathology severity and pathogenesis
Once again, I realize that the three letter agencies, major journals and academia have it all wrong. The whole point of the (I believe) Spike Protein bioweapon is to DESTROY THE MICROVASCULATURE and in so doing, induce SYSTEMIC FIBROSIS while simultaneously inducing ONCOGENESIS.
FURIN AND FIBROSIS
FURIN is implicated in the development and progression of fibrosis. For example, it is involved in the development of fibrosis after cardiac infarction (which is, of course, cardiac vasculature damage).
mRNA levels of Furin and TGF-β1 were elevated after infarction (p<0.05). With prolonged time periods of myocardial infarction, protein levels of Furin and TGF-β1 were further increased.
Role of miR-24, Furin, and Transforming Growth Factor-β1 Signal Pathway in Fibrosis After Cardiac Infarction
https://medscimonit.com/abstract/index/idArt/898641
It is also implicated in Cystic Fibrosis.
Here we measured activity of the major TGN (trans-Golgi network) endoprotease furin and demonstrated a marked upregulation in human CF cells. Increased furin activity was linked to elevated production in CF of the immunosuppressive and tissue remodeling cytokine TGF-beta and its downstream effects, including macrophage deactivation and augmented collagen secretion by epithelial cells. As furin is responsible for the proteolytic processing of a range of endogenous and exogenous substrates including growth factors and bacterial toxins, we determined that elevated furin-dependent activation of exotoxin A caused increased cell death in CF respiratory epithelial cells compared with genetically matched CF transmembrane conductance regulator-corrected cells. Thus elevated furin levels in CF respiratory epithelial cells contributes to bacterial toxin-induced cell death, fibrosis, and local immunosuppression.
Elevated furin levels in human cystic fibrosis cells result in hypersusceptibility to exotoxin A-induced cytotoxicity
https://www.jci.org/articles/view/31499
FURIN AND ONCOGENESIS
Squamous cell carcinoma (SCC) of the tongue is a common malignancy of the oral cavity. Furin convertase activates several precursor matrix metalloproteinases involved in the degradation of the extracellular matrix. The pattern of expression of furin and vascular endothelial growth factor-C (VEGF-C), two key molecules in neoplasm development, was examined during the progression from normal epithelium to invasive SCC. These findings suggest that furin is a useful marker of tumor progression and is responsible for VEGF-C processing. This in turn would enhance angiogenesis, leading to increased MVD associated with preinvasive and invasive neoplasia.
Simultaneous Expression of Furin and Vascular Endothelial Growth Factor in Human Oral Tongue Squamous Cell Carcinoma Progression
https://aacrjournals.org/clincancerres/article/10/13/4480/94509/Simultaneous-Expression-of-Furin-and-Vascular
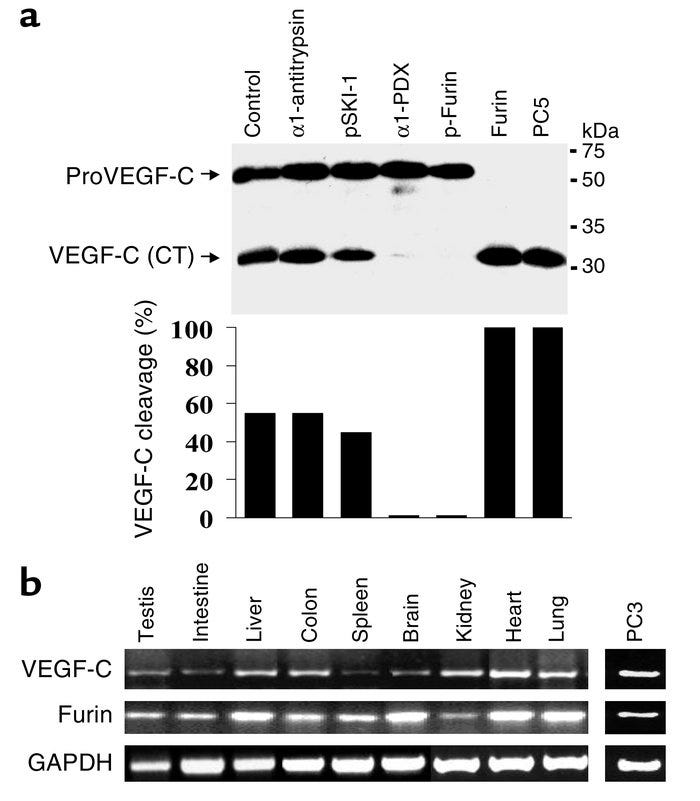
In conclusion, this study demonstrated that FURIN, PC5, and PC7 are the cognate PCs implicated in the dibasic cleavage and activation of proVEGF-C at the dibasic ArgArg227, and underlined the importance of this processing in VEGF-C–mediated functions. In this context, inhibition of the PC-mediated processing of proVEGF-C may provide a novel potential strategy in cancer therapy, in conjunction with other approaches such as using a soluble VEGFR-3 extracellular domain to inhibit tumor angiogenesis and lymphangiogenesis. VEGF-C expression has been detected in 50% of all the human cancers analyzed, and its expression has been correlated with lymph node dissemination in human cancers.
The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis
https://www.jci.org/articles/view/17220
Furin/PC processing of substrates has been shown to also contribute to tumour progression, aggressiveness, metastasis, and angiogenesis. Tumour invasion and metastasis represent a multistep process that depends on the activity of many proteins. Proteolytic degradation of the ECM components is a central event of this process.
The Role of Furin in the Development of Skin Cancer
https://www.intechopen.com/chapters/44255
THE FURIN FEEDBACK LOOP AND ONCOGENESIS
This is another very dangerous aspect of the Spike Protein. It triggers the Furin Feedback Loop as it induces TGF-B signaling.
Notably, positive feedback loops can further enhance the oncogenic potential of furin. For example, the furin substrate TGFβ not only increases furin mRNA expression, but also enhances its proteolytic activity by an unknown mechanism. Similarly, furin enhances the secretion of IFNγ, which in turn activates the FUR promoter.
Furin‐mediated protein processing in infectious diseases and cancer
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6682551/
Here, we report that cell-intrinsic interactions between the Spike (S) glycoprotein of SARS-CoV-2 and epithelial/endothelial cells are sufficient to trigger barrier dysfunction in vitro and vascular leak in vivo, independently of viral replication and the ACE2 receptor. We identify an S-triggered transcriptional response associated with extracellular matrix reorganization and TGF-β signaling.
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling
https://www.biorxiv.org/content/10.1101/2021.12.10.472112v1.full.pdf
GP120 AND FIBROSIS
However, evidence from autopsy studies performed in the “preprotease era” also demonstrated eccentric atherosclerotic lesions in the proximal coronary arteries and intimal fibrosis, in the absence of traditional cardiac risk factors.
HIV envelope glycoprotein gp120 (gp120) uses chemokine receptors CCR5 or CXCR4, usually with or rarely without the coreceptor CD4, to gain entry into target cells. In addition to facilitating viral entry, gp120 initiates signaling events that affect postentry stages of infection and modulate cellular functions, even in the absence of infection.
Smooth muscle cells (SMC) are the major cellular components of the arterial wall, and their proliferation and migration is key to the development of atherosclerotic plaques. Tissue factor (TF), the initiator of coagulation, is abundant in the plaque and in the injured arterial wall. Exposure of TF by plaque rupture may cause thrombosis and lead to acute myocardial infarction and unstable angina. Similarly, acute arterial injury, such as that produced during percutaneous coronary interventions, may also expose TF and promote thrombosis.
HIV envelope gp120 activates human arterial smooth muscle cells
https://www.pnas.org/doi/10.1073/pnas.181328798
This study shows that HIV gp120 modulates different aspects of HSC biology, including directional cell movement and expression of proinflammatory cytokines. These results identify a direct pathway possibly linking HIV infection with liver fibrogenesis via envelope proteins.
gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis
https://gut.bmj.com/content/59/4/513.abstract
GP120 AND ONCOGENESIS
HIV proteins, namely envelope protein gp120, accessory protein negative factor Nef, matrix protein p17, transactivator of transcription Tat and reverse transcriptase RT, are known to be oncogenic per se, to induce oxidative stress and to be released from the infected or expressing cells. These properties are proposed to underlie their capacity to affect bystander epithelial cells causing their malignant transformation, and to enhance tumorigenic potential of already transformed/cancer cells. HIV proteins can act alone or in collaboration with other known oncoproteins, specifically originating from the oncogenic human viruses such as human hepatitis B and C viruses, and human papilloma viruses of high carcinogenic risk, which cause the bulk of malignancies in people living with HIV-1 on ART.
Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind
https://escholarship.org/content/qt1rs1m9cn/qt1rs1m9cn.pdf
THE EFFECT OF FURIN AND GP120 ON THE ENDOTHELIUM
Two different full-length recombinant HIV gp120 proteins (HIV-1CM235 gp120 and HIV-1MN gp120) also induced neuronal and brain endothelial cell death, and concentrations as little as 1 ng/ml evoked pronounced morphological changes in these cells and marked cytotoxicity. This study suggests that HIV proteins and peptides that are shed in vivo may be directly involved in blood-brain barrier damage and neuronal cell death in HIV-associated dementia.
HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia
https://pubmed.ncbi.nlm.nih.gov/12430716/
HIV gp120 and TNF-α synergistically reduce eNOS expression and cause endothelial dysfunction in both porcine coronary arteries and HCAECs. ICAM-1 induced by TNF-α pretreatment may mediate HIV gp120-induced endothelial dysfunction, which suggests a novel molecular mechanism of HIV gp120–ICAM-1 interaction inducing endothelial dysfunction.
HIV gp120 induces endothelial dysfunction in tumour necrosis factor-α-activated porcine and human endothelial cells
https://academic.oup.com/cardiovascres/article/87/2/366/441475
The coronary artery disease risk variant at the 15q26.1 locus modulates FURIN expression in vascular ECs. FURIN levels in ECs affect monocyte-endothelial adhesion and migration.
FURIN Expression in Vascular Endothelial Cells Is Modulated by a Coronary Artery Disease-Associated Genetic Variant and Influences Monocyte Transendothelial Migration
https://pubmed.ncbi.nlm.nih.gov/32067586/
We show that systemic inhibition of FURIN in mice decreases vascular remodeling and atherosclerosis. FURIN-mediated modulation of MMP2 activity may contribute to the atheroprotection observed in these mice.
FURIN Inhibition Reduces Vascular Remodeling and Atherosclerotic Lesion Progression in Mice
https://www.ahajournals.org/doi/10.1161/ATVBAHA.118.311903
What I have endeavored to demonstrate in this post is that the FURIN CLEAVAGE SITE and the GP120 INSERT are TWO SIDES OF THE SAME FIBROTIC/ONCOGENIC COIN that the Spike Protein of SARS-CoV-2 is. All of this fits in perfectly with my SPED hypothesis and leads me to believe that the Endothelium is but a first step of a complete “invasion” of the body. It may be that all organs may be subject to the same damage that the Endothelium suffers from the Spike Protein. It may also be that the microvascular destruction alone, once it reaches the major organs is sufficient to cause mortality.
I still believe that there is a “tipping point” with regards to SARS-CoV-2/Spike Protein exposure and the development of SPED. I currently do not believe that one exposure is enough to induce fatal SPED (long term) in all. It is obvious that in a small subset of people, one exposure does cause the induction of fatal SPED.
Is FURIN EXPRESSION the DETERMINANT OF SPED (AND COVID!) MORTALITY?
Does FURIN EXPRESSION change after repeated exposures to the Spike Protein?
These are questions to which we certainly need immediate answers.



Walter, you are better than so many doctors! You have a heart of gold, what a gift to humanity!! You will have a special place in heaven!! 🙏🏻❤️
Nice work mate, I can see the round table (zoom) meeting has got you thinking about the furin cleavage site.
It was great to hear you speak, keep up the hard work. 🙏🏻