Systemic Microvascular Disease: Stage II of SPED (Spike Protein Endothelial Disease)
Once the microvasculature has been “seeded” with the Spike Protein, systemic microvascular dysfunction ensues in a paracrine manner.
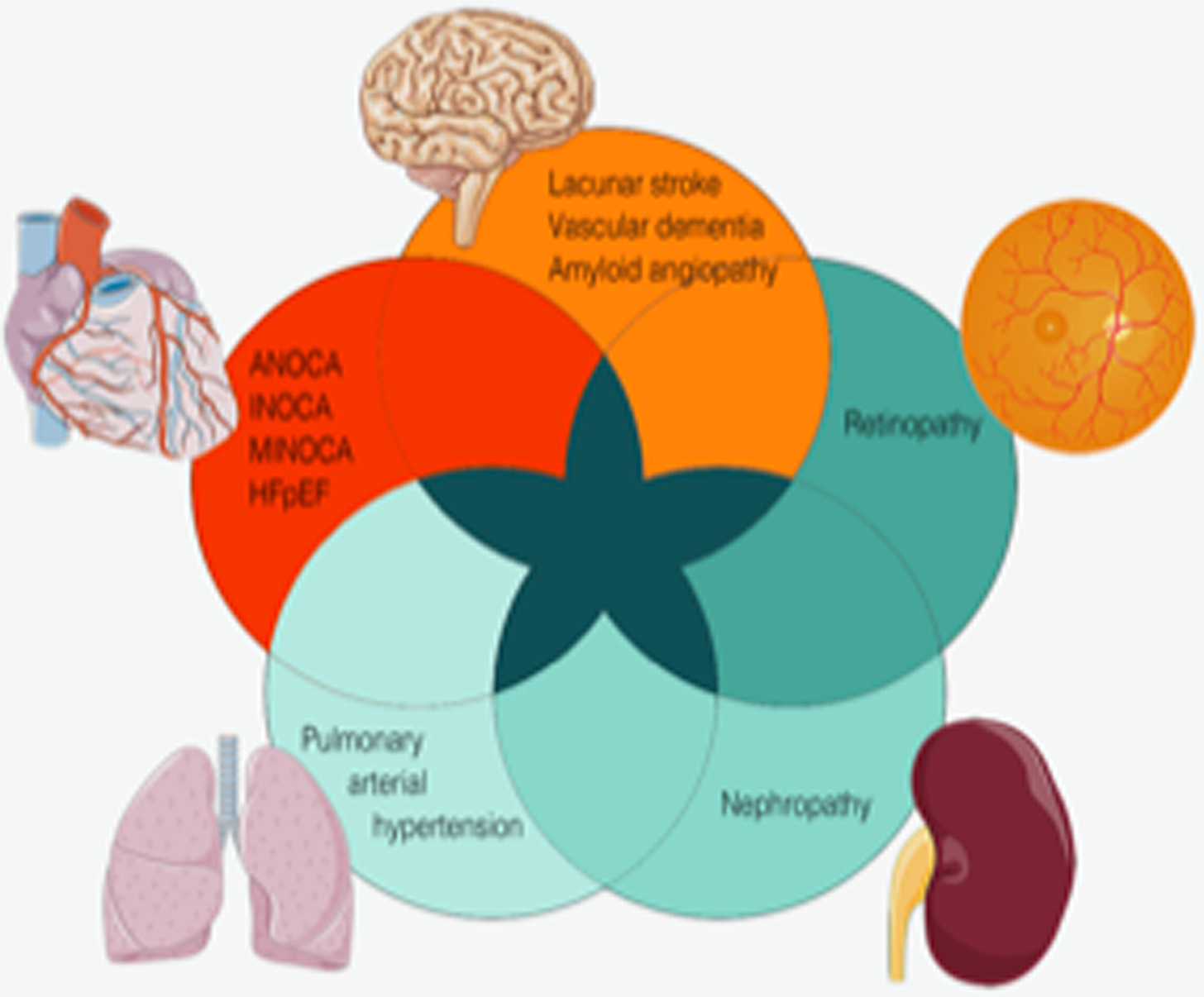
Microvascular dysfunction affecting the heart, brain, retina, lung, and kidney, representing different manifestations of small-vessel disease. They share pathophysiologic mechanisms and can occur concomitantly. ANOCA, angina with no obstructive coronary artery disease; HFpEF, heart failure with preserved ejection fraction; INOCA, ischemia with no obstructive coronary artery disease; MINOCA, myocardial infarction with no obstructive coronary arteries.
Now that it has been almost five years since the emergence of SARS-CoV-2 and its Spike Protein, we can observe a pattern of long-term damage from the Spike Protein. The long-term effects have been called everything from Long COVID, to PASC to Vaccine Injury. However, if we look very closely, a picture begins to emerge that is the picture of a novel systemic microvascular disease.
That the Spike Protein causes microvascular dysfunction and inflammation is well proven.
In addition to the implications of ACE2 downregulation, our observations also point to a novel effect of spike protein on endothelial cell function and stability. We documented that the engagement of spike protein reduces surface ACE2 and intracellular KLF2 expression, while increasing cell surface expression of vWF in primary human arterial endothelial cells. The changes in KLF2 and vWF expression are indicative of endothelial dysfunction and may perpetuate vascular inflammation and coagulation.
SARS-CoV-2 Spike Protein Destabilizes Microvascular Homeostasis
https://journals.asm.org/doi/10.1128/spectrum.00735-21
Looking at Long COVID/Spike Protein injury we notice manifestations of systemic microvascular disease.
Fatigue, chest pain, and neuro-cognitive difficulties were significantly associated with endothelium dysfunction with an EQI <2 after adjustment for age, sex, diabetes, hypertension, dyslipidemia, coronary heart disease, and the severity of acute COVID-19 infection. In multivariate analysis, endothelial dysfunction (EQI <2), female gender, and severe clinical status at acute COVID-19 infection with a need for oxygen supplementation were independent risk factors of long COVID-19 syndrome. Long COVID-19 symptoms, specifically non-respiratory symptoms, are due to persistent endothelial dysfunction.
Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study
https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2021.745758/full
We find this dysfunction if we look at the hearts of those with Long COVID
First-pass stress perfusion CMR showed a significant circumferential subendocardial perfusion defect in 5 patients (50%), highly suggestive of microvascular dysfunction. In all patients, epicardial artery disease was ruled out by coronary CT angiography.
Long COVID-19 and microvascular disease-related angina
https://www.revespcardiol.org/en-long-covid-19-microvascular-disease-related-angina-articulo-S1885585721003224
And in the brain.
SARS-CoV-2 infection may induce abnormalities in innate and adaptive immunity including monocyte expansion, T-cell exhaustion, and prolonged cytokine release, which may cause neuroinflammatory responses and microglia activation, white matter abnormalities, and microvascular changes. Additionally, microvascular clot formation can occlude capillaries and endotheliopathy, due to SARS-CoV-2 protease activity and complement activation, can contribute to hypoxic neuronal injury and blood–brain barrier dysfunction, respectively.
Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10001044/
Finally, overall, systemically. Please note this paper reflects findings readers will recognize that I made years ago. As I predicted, this microcirculatory pathology progresses.
Vascular sequelae following (SARS-CoV-2 coronavirus disease) (COVID)-19 infection are considered as “Long Covid (LC)” disease, when occurring 12 weeks after the original infection. The paucity of specific data can be obviated by translating pathophysiological elements from the original Severe Acute Respiratory Syndrome-Corona Virus (SARS-CoV-2) infection (In a microcirculatory system, a first “endotheliitis,” is often followed by production of “Neutrophil Extracellular Trap,” and can evolve into a more complex leukocytoklastic-like and hyperimmune vasculitis. In medium/large-sized vessels, this corresponds to endothelial dysfunction, leading to an accelerated progression of pre-existing atherosclerotic plaques through an increased deposition of platelets, circulating inflammatory cells and proteins.
Vascular “Long COVID”: A New Vessel Disease?
https://journals.sagepub.com/doi/10.1177/00033197231153204
Yet, what could the mechanism ultimately be? I propose that it is paracrine signaling induced by the Spike Protein. Endothelial cells which are dysfunctional can cause neighboring endothelial cells to, also, become dysfunctional. And so create a cascade.
Along with the local effects of this transition in the adipose tissue microenvironment, dysfunctional endothelial cells were able to produce and release increased number of extracellular vesicles with inflammatory and immune activities, which might be disseminated in a paracrine or endocrine manner to cause endothelial dysfunction in remote vascular beds.
Coronary Microvascular Dysfunction
https://www.ahajournals.org/doi/10.1161/ATVBAHA.121.316025#F2
It is important to note that finding this and ultimately proving its existence is not an easy task. The greatest problem is that we have limitations in visualizing the microvasculature. This is changing as super-resolution ultrasound microvascular imaging is being readied for clinical use.
Therefore, the accurate diagnosis of microvascular angina necessitates the exclusion of obstructive CAD. However, due to the current limitations in directly visualizing the coronary microvasculature, diagnostic measures for CMD mainly rely on surrogate markers of myocardial perfusion and microvascular resistance.
Coronary microvascular dysfunction, arrythmias, and sudden cardiac death: A literature review
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10998042/
The selection of the above paper is intentional. Yes, this type of dysfunction can cause arrythmias and sudden cardiac death. I highly recommend reading the paper in its entirety. It discusses essential coronary implications of a Spike Protein-induced microvascular disease.
Now for the questions:
Does this process initiate in all exposed to the Spike Protein?
Do some clear the Spike and repair the damage?
Is the damage cumulative? Does each exposure to the Spike Protein progress this process?
Are there those which are immune to the Spike?
What does the microvasculature of those exposed to COVID/Spike Protein therapies/Long COVID look like?
We need answers to the above questions to begin to have an understanding of the scope of which we are dealing with.
I will continue to research mechanisms and investigate therapeutics. I cannot emphasize enough how much I need and appreciate your support. Thank you, as always for that support, dialogue and readership.



Questions 2 & 4, I believe are related to blood type. Type O seems to be the most resistant from my observations.
I know this might be out of line, but here goes. I need help. I was hospitalised with what was called covid, and since have suffered from what I am self-diagnosing as long covid. As part of that, my blood pressure has skyrocketed, and my doctor has me on ACE inhibitors (Candesartin), which are not working and for which he keeps increasing the dose in the hope that a higher dose will achieve something more. We are close to the 32mg maximum with no effect. I am also taking 10mg of Lercanidipine to no effect. He does not acknowledge the existence of long covid and so I cannot talk to him about the potential spike protein/ACE2 receptor problem with the Candesartin. (Don't suggest I change doctor, he is number 7 and is at least willing to support my decisions even when he does not understand them.)
Can you tell me whether taking ACE inhibitors is going to make the potential spike protein/ACE2 receptor problem worse? If it is, I will get him to switch me to a different medication while I back out of the ACE inhibitor slowly and hopefully safely. (Note that no number of different natural blood pressure medications are having the slightest impact on my steadily high numbers either (systolic is from the high 150s to low 170s routinely and flaring under extreme stress up to 200, and diastolic in the 80s-90s, only very rarely over 100). I have had all sorts of scans and blood test to find constrictions and/or blood abnormalities, to no effect.