PRRARSV – The Furin Cleavage Site: A Nuclear Localization Signal that Translocates the Spike and its mRNA to the Nucleus Inducing H3.3 Histone Deposition and Rapid Aging
Why the Furin cleavage site, not found in other Cornaviruses, is far, far more (and more dangerous) than just a cleavage site.
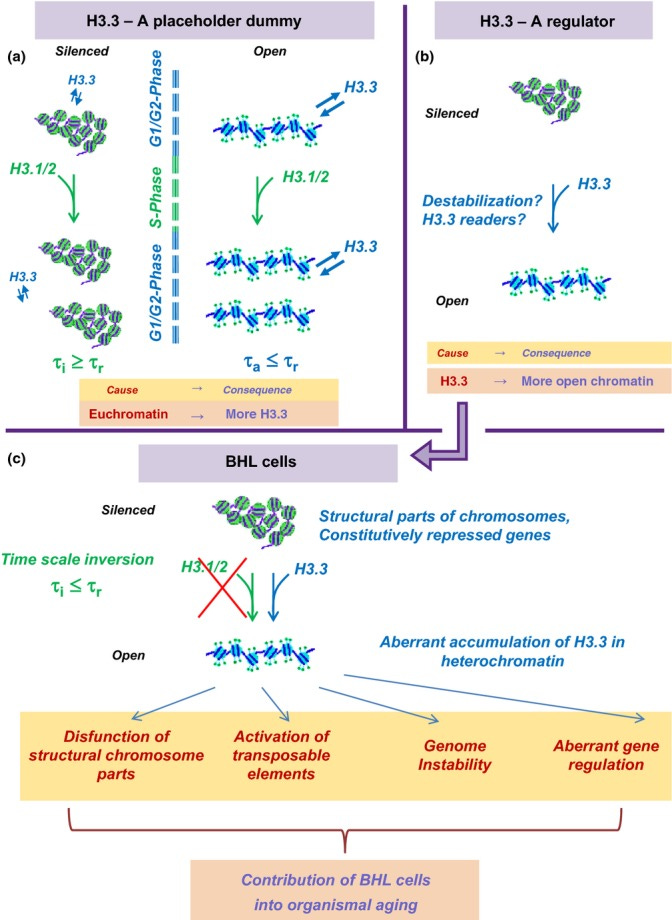
H3.3 Dilemma. Top. Alternative explanations for association of H3.3 with active chromatin. (a) A placeholder dummy. Euchromatin (right) is more open and more prone to histone damage and exchange than heterochromatin (left). Time of turnover of H3 histones in active chromatin τa is shorter than time of cell replication τr. Accordingly, replication-coupled deposition of canonical H3.1/2 cannot be responsible for the turnover of all H3 histones, which is expected to lead to a preferential accumulation of H3.3 in open chromatin. To the contrary, the turnover rate of H3 in heterochromatin τi is slower than τr, and the replication-coupled H3.1/2 deposition can be sufficient for H3 replacement. (b) A regulator. H3.3-containing chromatin is ‘special’ in some respect, and H3.3 replacement of the canonical H3.1/2 leads to chromatin opening or other consequences for its structure and function. Down. Consequences of the ‘regulator’ model in the case of below Hayflick limit (BHL) cells. The replication time of BHL cells is expected to be slower than the rate of H3 exchange in heterochromatin, which should lead to an accumulation of H3.3 histones in heterochromatin of BHL cells with potentially negative consequences in terms of structure and function.
The now infamous Furin Cleavage Site of the Spike Protein of SARS-CoV-2 is far more than just a cleavage site. This Cleavage Site is also a Nuclear Localization Signal (NLS). It instructs the cell to transport itself into the nucleus of the cell. There, I will show, it then induces H3.3 Histone Deposition resulting in rapid aging of the organism, explaining the vast array of age-related diseases we are observing post COVID infection and Spike Protein exposure.
A paper was published on September 27, 2022 which confirmed that the Spike Protein and its mRNA is being translocated to the nucleus of the cell. There, I will show how it is almost certainly inducing the deposition of Histone H3.3 into the chromatin, causing rapid aging of the organism.
In conclusion, the SARS-CoV-2 S protein has a functional pat7 NLS “PRRARSV”, that results in one out of four S proteins translocating into the nucleus in infected cells. S Protein appears to shuttle S mRNA (possibly the genome) into the nucleus as well. Thus, the NLS of the S protein may contribute to the evasion of the host immune response and is a novel pathogenic feature of SARS-CoV-2.
Nuclear translocation of spike mRNA and protein is a novel pathogenic feature of SARS-CoV-2
https://www.biorxiv.org/content/10.1101/2022.09.27.509633v1.full
Yet, what the paper failed to discuss is that once the Spike Protein has translocated to the nucleus, the S2 subunit interacts with Histone H3.
What I believe is occurring is that the interaction of the S2 unit with Histone H3, along with the Spike Protein’s induction of the Heat Shock Response, is inducing deposition of H3.3. This is then having catastrophic consequences for the host – especially in terms of aging and age-related disease. It would appear that the body views the assault of the S2 subunit on the H3 histone as removing or disassembly of the Histone, therefore calling in the replacement H3.3.
Deposition of variant histones requires the removal or disassembly of existing nucleosomes.
The use of tagged variants allowed us to monitor nucleosomal abundance of specific H3 variants during and following a temporary induction of a heat-shock-responsive gene (HSP70).
Variant histone H3.3 is deposited at sites of nucleosomal displacement throughout transcribed genes while active histone modifications show a promoter-proximal bias
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1182337/
The second, Hsp70, has been implicated in host cell recognition of SARS-CoV-2 and viral entry. Since the dawn of COVID-19, initial studies have shown that GRP78 is overexpressed in the endoplasmic reticulum (ER) destined for the cell membrane. Both predictive and structural studies have shown that GRP78 assists in the host cell recognition of SARS-CoV-2 spikes. The binding of Grp78 to a C480-C488 (CNGVEGFNC) region on the C-terminus of S1 (Spike) is predicted to occur via hydrogen and hydrophobic interactions.
The capture of host cell’s resources: The role of heat shock proteins and polyamines in SARS-COV-2 (COVID-19) pathway to viral infection
https://www.degruyter.com/document/doi/10.1515/bmc-2022-0008/html?lang=en
Now we arrive at the point. The Furin Cleavage Site, by ultimately inducing H3.3 deposition, is having extremely pathological effects on the host.
Now, let us consider that the association of H3.3 with active chromatin is due to its role in establishing an active and open chromatin state. In this case, one should expect that its incorporation into heterochromatin could affect BHL cells in an undesirable way. Heterochromatin-based silencing is an essential mechanism employed to restrict gene expression to housekeeping and lineage-specific genes, as well as to suppress parasitic selfish elements (e.g., transposons). Repetitive sequences with a structural role (such as satellite DNA) need also be transcriptionally silenced. One can see how the opening of otherwise silenced chromatin in inappropriate contexts would lead to unwanted transcriptional activation (or else competition with other genomic sites for binding of available transcription factors) with negative consequences due to perturbed epigenetic programs and induction of genome instability (via activation of transposable elements and/or affecting structural parts of the chromosomes).
The biological role of H3.3 has been the subject of intensive recent research. Adding more urgency to this effort, increasing evidence implicates H3.3 and its chaperones in cancer (Schwartzentruber et al., 2012; Behjati et al., 2013; Fontebasso et al., 2013; Aihara et al., 2014; Venneti et al., 2014).
Thus, the H3.3 dilemma considerations do pertain to the biology of adult SC, especially during their nonreplicating stage, even though they are little affected by the Hayflick limit. Despite many technical challenges in isolating and working with dormant adult SC, future research should shed light on how the accumulation of H3.3 in heterochromatin could affect their main characteristics: capacity to self-renew and differentiate, which is directly relevant to organismal aging.
More importantly, changes in the gene expression profiles due to H3.3 misincorporation can help to explain how cellular senescence contribute to organismal aging – in ways that come in addition to the mere loss of proliferation potential and limited tissue regeneration. One of the marks of cellular senescence is senescence-associated secretory phenotype (SASP), which results in the secretion of various growth factors, cytokines, and proteases (called summarily senescence messaging secretome (SMS) (Kuilman & Peeper, 2009)), leading to age-related tissue dysfunction and disruption (Coppe et al., 2008; Rodier & Campisi, 2011).
Improper H3.3 accumulation in heterochromatin could activate otherwise silenced genes and synergize with the negative effect of unrepaired DNA lesions which accumulate therein. This is another facet of the H3.3 dilemma that awaits further investigation.
Molecular turnover, the H3.3 dilemma and organismal aging (hypothesis)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4406661/
This demonstrates how the Furin Cleavage Site, in a very clever fashion, actually functions not so much to cleave the Spike, but to bring it into the nucleus. There it functions in perhaps its most powerful way to induce rapid aging of the host.



ouch, this research really does concern me on the spike and its malevolence. It really does look designed to kill.
It'd be great to have a "Walter Chestnut for Dummies".