Permanent Endothelial Activation: The Spike Protein and Cell Adhesion: Understanding Why What Appears to be an Endothelial Disease is Actually a Cell Adhesion Disease
Explaining how Spike Protein Endothelial Disease is the first phase of a Cell Adhesion Disease, inducing autoimmune diseases (like diabetes), “transplant rejection” and cachexia.
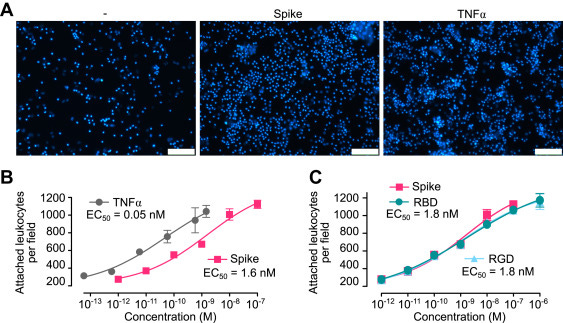
Spike-induced leukocyte adhesion to EC. A, micrographs showing leukocytes adhered to HUVEC monolayers incubated in the absence (−) or the presence of spike (10 nM) or TNF⍺ (1 nM). The scale bar represents 120 μm. Dose–response stimulation of leukocyte adhesion to HUVECs in response to spike, TNF⍺. B, the receptor-binding domain of spike (RBD) or the RGD tripeptide. C, values are means ± SD, n = 9. Dose–response curves were fitted by least squares regression analysis (r2 > 0.93). EC, endothelial cell; HUVEC, human umbilical vein endothelial cell; RGD, arginine–glycine–aspartic acid; TNFα, tumor necrosis factor alpha.
This has been a grueling, arduous and tedious process unraveling what is actually happening when the Spike Protein invades the body. I continue to work as I believe we have not yet hit Ground Zero. However, today I believe we are much, much closer to understanding exactly what is happening.
First, ACE-2 as we now know, is not, by far, the only receptor that the Spike Protein can attach to. It can also attach to integrin ⍺5β1 in endothelial cells.
We suggest that the spike protein, through its RGD motif in the receptor-binding domain, binds to integrin ⍺5β1 in ECs to activate the NF-κB target gene expression programs responsible for vascular leakage and leukocyte adhesion.
Furthermore, this attachment initiates an expression cascade.
Incubation of human umbilical vein ECs with whole spike protein, its receptor-binding domain, or the integrin-binding tripeptide RGD induced the nuclear translocation of NF-κB and subsequent expression of leukocyte adhesion molecules (VCAM1 and ICAM1), coagulation factors (TF and FVIII), proinflammatory cytokines (TNFα, IL-1β, and IL-6), and ACE2, as well as the adhesion of peripheral blood leukocytes and hyperpermeability of the EC monolayer. In addition, inhibitors of integrin ⍺5β1 activation prevented these effects. Furthermore, these vascular effects occur in vivo, as revealed by the intravenous administration of spike, which increased expression of ICAM1, VCAM1, CD45, TNFα, IL-1β, and IL-6 in the lung, liver, kidney, and eye, and the intravitreal injection of spike, which disrupted the barrier function of retinal capillaries.
The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling
https://www.jbc.org/article/S0021-9258(22)00135-1/fulltext
So, what does this mean? It means that much of what follows an acute SARS-CoV-2 infection (Long COVID), or Spike Protein exposure, can be explained as a dysregulation of cell adhesion, initiating in the Endothelium.
THE ENDOTHELIUM MAY BECOME PERMANENTLY ACTIVATED BY THE SPIKE PROTEIN
Let us look at what the above integrin signaling can induce.
One of the most potent activators of the endothelium is TNF, which can also be expressed by endothelial cells, causing a permanent, autocrine stimulatory signal… Adult tie2-transmembrane TNF-transgenic mice developed chronic inflammatory pathology in kidney and liver, characterized by perivascular infiltration of mononuclear cells into these organs. Along with the infiltrate, an up-regulation of the adhesion molecules ICAM-1 and VCAM-1, but not E-selectin, in the endothelium was observed.
Tumor necrosis factor is a pleiotropic, proinflammatory cytokine, mainly produced by activated macrophages and T cells. It is involved in tissue remodeling, defense against infection, and inflammation and can induce necrosis in certain experimental tumors. Furthermore, it has been directly implicated in severe pathological situations, such as septic shock, cachexia, graft-vs-host diseases, and the autoimmune disorders multiple sclerosis, Crohn’s disease, diabetes, and rheumatoid arthritis.
Chronic Inflammation and Protection from Acute Hepatitis in Transgenic Mice Expressing TNF in Endothelial Cells
https://journals.aai.org/jimmunol/article/167/7/3944/83552/Chronic-Inflammation-and-Protection-from-Acute
And, indeed, this very infiltration of leukocytes by the Spike Protein creates a FEEDBACK LOOP! Spike induces expression of adhesion molecules, leukocytes come to clear it out, Spike induces expression of adhesion molecules…
We recently reported persisting elevation of a triad of monocyte/macrophage-related cytokines—interleukin-1β (IL-1β), IL-6, and tumor necrosis factor (TNF) 8–10 months after SARS-CoV-2 infection in patients with PASC.6 We hypothesized that these cytokines are secreted by tissue-resident macrophages that engage into a self-sustaining pro-inflammatory loop that may fuel PASC. Such macrophage imprinting has been previously reported to be potentially induced through engulfment of spike protein by tissue-resident macrophage in early disease phases.
Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19
https://onlinelibrary.wiley.com/doi/10.1002/jmv.28364
THEREFORE!
The body must either be unable to clear the Spike Protein (inability to break it down) or, the Spike Protein is being constantly produced, or both.



Thank you Walter. "The body must either be unable to clear the Spike Protein (inability to break it down) or, the Spike Protein is being constantly produced, or both." - let's say that the body cannot clear the spike. Your earlier stacks suggest that there are natural substances that can clear the spike, like Nattokinase - correct? We need to disrupt the feedback loop if it exists in our bodies. Thanks again! May God bless you and continue to guide you. Peace.
I am always amazed by the work you are doing. While far above my education your explanations are helping me to understand. Thanks for hat you do!