Friday Hope: Stem Cells: Evidence for Successful Treatment in Acute COVID
Stem Cells also appear to greatly benefit those with Long COVID/Spike Pathologies.
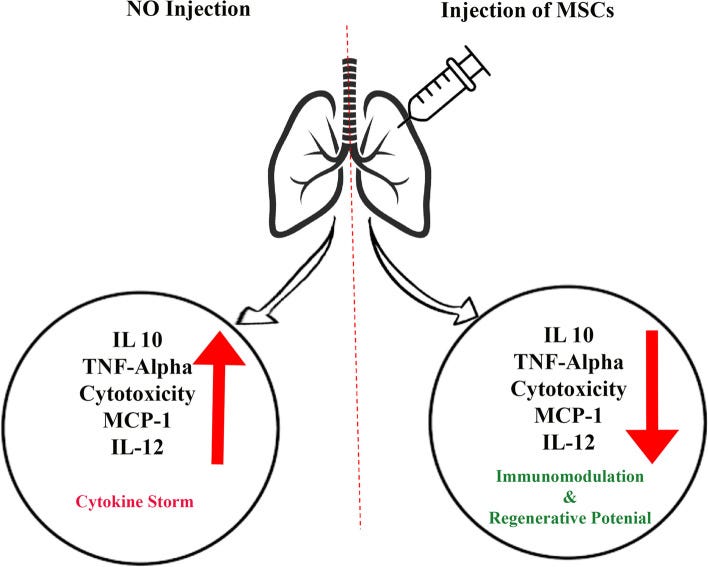
Effects of Mesenchymal stem cells (MSCs) injection on the patient’s lung. Injection of this cell reduces the secretion of interleukins 10, 12 and MCP-1. Reducing the secretion of these inflammatory factors prevents lung and respiratory diseases such as COVID-19.
Reviewing all the evidence we have seen regarding the Spike Protein over the years, one truth is very apparent: The Spike Protein has the ability to either kill or permanently alter cells. Therefore, it is only logical that replacing dead or altered cells with healthy, new cells should help the body recover from COVID and Spike Protein-related pathologies. Indeed, this appears to be the case.
First, let us review how Stem Cells are effective in treating the acute phase of COVID.
SARS-inflammatory Cov-2’s response is the primary method for destroying the virus, but this activity damages and dysfunctions the body’s tissues. Viral entrance into tissue results in the release of pro-inflammatory molecules such as IL-1, IL-17, TNF-, and INF-.
Newly, stem cell therapy has become an excellent approved tool for treating viral lung damage. Because attempts to treat lung damage with a variety of drugs have not been successful, the use of cell therapy has been suggested. MSCs have a high ability to repair and regenerate.
MSC-based treatments also showed encouraging results in the experimental therapy of lung failure by reducing alveolar collapse, collagen buildup, and cell death in the tissue.
Stem cell therapy for COVID-19 pneumonia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8850486/
In a very large meta-analysis of Stem Cell therapy in COVID the results were affirming. This study was headed by Otavio Cabral-Marques and published in September of this year. Noteworthy is the following:
Stem cell therapy and models involving organoids derived from stem cells drew a great deal of attention as novel methods for treating and studying COVID-19 during the pandemic, he noted, given the significant immune regulatory power and tissue repair functions of stem cells, especially the mesenchymal variety. In the case of the lungs, for example, clinical trials have shown to a greater or lesser extent that advanced cell therapy can limit the severity of the inflammatory response in COVID-19 patients, reduce pulmonary damage, improve lung function and help combat fibrosis.
Stem cell therapy shows promise in treating COVID-19 complications
https://www.news-medical.net/news/20230919/Stem-cell-therapy-shows-promise-in-treating-COVID-19-complications.aspx
Systematic review and meta-analysis of cell therapy for COVID-19: global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10321603/
The ability of Stem Cells to successfully treat Acute COVID is great news in and of itself. However, when you realize that Stem Cells also have the ability to treat those with Long COVID/Spike Protein pathologies, there is suddenly great hope for those who have been burdened with long term and debilitating injury and disease.
Results
VAS scores and Fatigue Assessment scores (FAS) showed significant improvements post-treatment (P = 0.0039, ES = 0.91) compared to baseline. Respiration rates and oxygen saturation levels that were within the normal range at the baseline remained unchanged at the end of the study (EOS). Paired comparison between baseline and EOS for short-form-36 health survey questionnaire (SF-36) scores also showed improved quality-of-life with significant improvements in individual SF-36 evaluations. Mostly mild AEs were reported during the study period with no incidence of serious AEs. Also, no detrimental effects in laboratory values were seen.
Conclusions
The results of the expanded access program indicated that treatment with autologous HB-adMSCs resulted in significant improvements in the signs and symptoms associated with post-COVID-19 syndrome as assessed by VAS and FAS scores. Additionally, improvements in the patients’ quality-of-life as demonstrated using SF-36 scores that also showed significant improvements in individual scaled scores. Overall, administration of multiple infusions of autologous HB-adMSCs is safe and efficacious for improvements in the quality-of life of patients with post-COVID-19 syndrome.
Adipose-derived, autologous mesenchymal stem cell therapy for patients with post-COVID-19 syndrome: an intermediate-size expanded access program
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10557203/
I am hopeful that the Spike Protein can ultimately be defeated. It will take care and vigilance, but the path forward seems ever clearer: Remove and Repair. If we can rid our bodies of the Spike Protein (and all genetic material which encodes for it) then repair the damaged tissues, we may restore ourselves to the healthy homeostasis we enjoyed before the introduction of this, to me at least, most unnatural guest.
As always, thank you for your continued readership and support. I wish everyone a most Merry Christmas and Happy New Year! Blessings and joy to all with deeply felt thanks and gratitude.



Where I live a stem cell infusion is $6000.00 Kevin McCairn mentioned that an infusion in Japan is about $500.00. So, for $6000 You get the infusion plus a nice trip to Japan, nice hotel, best sushi, see the sights, and have money left over.
Thank you Walter Chestnut! Have a Blessed and Berry Christmas. Thank you for all you are doing for humanity. Peace.