Turbocancers: A Secondary Manifestation of the Spike Protein’s Invasion of the Extracellular Matrix (ECM)?
Looking more closely at the mechanisms of the ECM and cancer, the Spike Protein may be initiating cancer and hyperaccelerating what is already there.
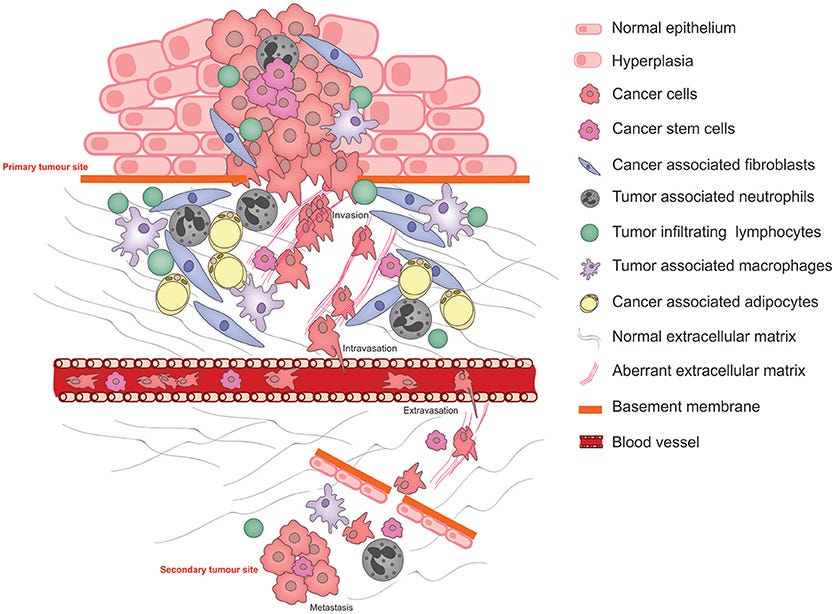
Schematic illustrating the pathophysiological processes that exploit cancer cell plasticity during tumor progression, invasion, and metastasis.
The explosion of turbocancers has become a major concern, not only in the medical community, but also for the general population. Almost all of us are by now unfortunately familiar with the news that a friend or family member died from a case of hyperaggressive (turbo) cancer. This was not so common a case before the appearance of the Spike Protein.
Readers of this Substack know that I have recently detailed how the Spike Protein continues its pathological journey after invading the endothelium by then invading the extracellular matrix. I outlined several consequences of this, mainly focusing on fibrogenesis.
I did touch upon the subject of turbocancers, indicating the feedback loop between fibroblasts and cancer cells. However, how does this occur? The answer appears to be the Spike Protein interfering with ECM components – and creating hyperinflammation.
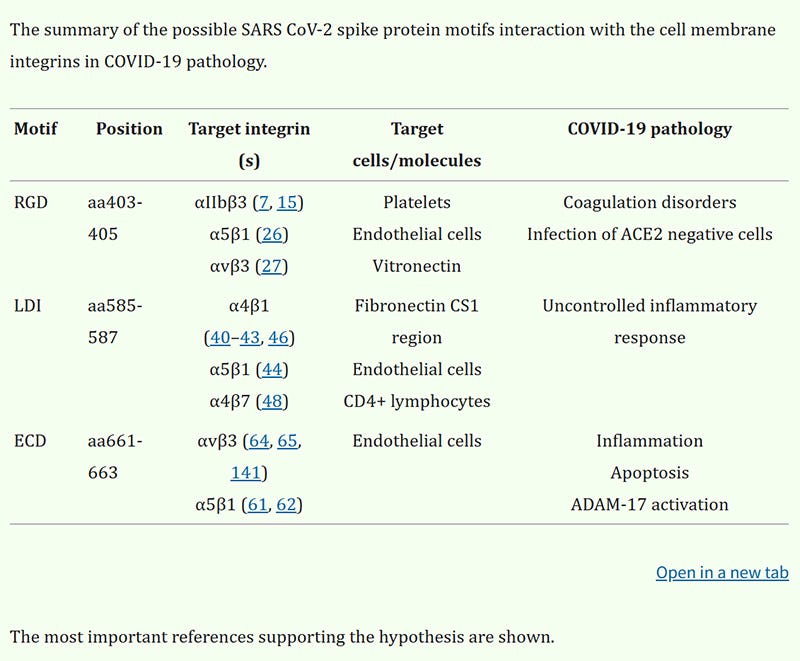
First, let us observe how the Spike Protein not only interacts with the endothelium, but also the ECM (Fibronectin).
So, not only do we have an uncontrolled inflammatory response from the Spike’s interaction with fibronectin, but we can also determine another deleterious function of the interaction. Please note how the Spike interacts with the CS1 region of fibronectin. Why is this important? Because this peptide actively inhibits tumor metastases! This is an important piece of the turbocancer explosion puzzle.
The connecting segment 1 (CS-1) is a cell attachment domain located in the type III homology connecting segment (IIICS) of fibronectin. CS1 peptide of fibronectin, which lacks the Arg-Gly-Asp-containing domain, actively inhibits tumor metastases in spontaneous and experimental metastasis models. The use of Fibronectin CS1 Peptide might offer a promising therapeutic approach for combating and preventing cancer metastasis.
Fibronectin CS1 Peptide
https://www.genscript.com/peptide/RP10838-Fibronectin_CS1_Peptide.html
Then we have the inflammatory component. Chronic inflammation is certainly linked to cancer initiation. Please note the presence of the all too familiar Spike-induced cytokines IL-6 and TNFa.
The inflammation which may promote cancer initiation is induced and exists long before tumor formation [112]. IBD, chronic hepatitis, Helicobacter-induced gastritis or schistosoma-induced bladder inflammation are correlated with an increased risk of colorectal cancer (CRC), liver, stomach and bladder cancers, respectively [113]. Chronic inflammation responds to one of the main recognized predisposing factors to cancer initiation, which is the accumulation of mutations in healthy cells [114]. Notably, inflammatory cells such as neutrophils and macrophages are the major producers of reactive oxygen species (ROS) and reactive nitrogen intermediate (RNI) species, which increase mutagenesis, predisposing to accumulation of mutations in normal tissue [112]. For instance, chronic intestinal inflammation is correlated with the accumulation of mutation in Tp53 or other cancer-related genes in intestinal epithelial cells [115,116,117,118]. Inflammatory cytokines are also correlated with predisposing cancer mutations. IL-22 induces the expression of DNA damage response genes to repair the genotoxic insult caused by inflammation [119]. Other signaling cytokines such as IL-6, TNF-α and IL-1 activate epigenetic machinery in epithelial cells, including DNA histone modification components (Dnmt1, Dnmt3, DOTL1), miRNA and lncRNA, modulating expression levels of oncogenes and tumor suppressors [120].
The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation?
https://pmc.ncbi.nlm.nih.gov/articles/PMC9741172/#sec3-cancers-14-05903
Also, the ECM, of course, signals cells in organs it is connected to. This results in transcription dysregulation that may lead to the development of cancer. Especially when you have signaling induced by hyperinflammation.
Therefore we could envision that signaling controlled by the ECM should lead gene transcription control. However, the availability of DNA sequences to which the transcription factors bind might be determined by the local nuclear environment, hence potentially making nuclear organization the cornerstone of gene expression control. The answer to this power dilemma might be in the cell matrix that provides a way to envision the integrated contribution of the ECM and nuclear organization to gene expression control.
Contributions of extracellular matrix signaling and tissue architecture to nuclear mechanisms and spatial organization of gene expression control
https://pmc.ncbi.nlm.nih.gov/articles/PMC2728154/#S7
So, we may now understand how the Spike Protein induces cancer through the ECM. After all, severe acute hyperinflammation may have the same effect on carcinogenesis as prolonged low-level inflammation. But, what of existing cancers?
This is also where the Spike Protein plays a crucial role in the recent onset of turbocancers. The Spike hyperaccelerates what is already there by interacting with the ECM. It is important to understand how long a tumor may exist before being detected.
To exemplify a realistic scenario in which biomarker is shed by both cancerous and noncancerous cells, we primed the model on ovarian tumor growth and CA125 shedding data, for which tumor growth parameters and shedding rates are readily available in published literature. We found that a tumor could grow unnoticed for more than 10.1 years and reach a volume of about π/6(25.36 mm)3, corresponding to a spherical diameter of about 25.36 mm, before becoming detectable by current clinical blood assays.
Mathematical Model Identifies Blood Biomarker–Based Early Cancer Detection Strategies and Limitations
https://www.science.org/doi/10.1126/scitranslmed.3003110
Yes, you read that correctly. Over ten years. Therefore, it is not surprising that the Spike Protein can “root out” those extant microtumors and cause them to “blossom.” I created a graphic years ago when I saw the Spike Protein causing metastases but had not yet fully defined the mechanisms. It was a satirical image of Miracle-Gro plant food – edited to be for tumors instead of plants.
I will continue to work on therapeutics to counter these pathological effects of the Spike Protein. The more we understand, the more we can mitigate. Thank you, as always, for your readership, dialogue and support.



I love your dedication and research, I’ve been following you for a few years, found you on twitter.
There’s a couple things different that no one is taking about, aside from the Covid.. is the antidote.. the novel vaccine. I’m wondering why this isn’t part of a study?
Could it be that younger generations are getting more cancer due to the lockdowns and explosion of wireless radiation they've exposed themselves to, in combination with the metals in the injections?
Before the age of electricity, cancers were rare, and brain cancer exploded with the introduction of cellular tech:
https://romanshapoval.substack.com/p/brain