The Spike Protein’s Downregulation/Mimicry of GABA May Exacerbate Spike Protein Endothelial Disease (SPED) and Induce Autoantibodies: The Interference With GABA Signaling via Endothelial Interaction
Celine Dion’s Recent Stiff Person Syndrome Diagnosis May Be Related – As Well As the Recent Surge in Non-Myocarditis Sudden Deaths via the Mechanism of SUDEP
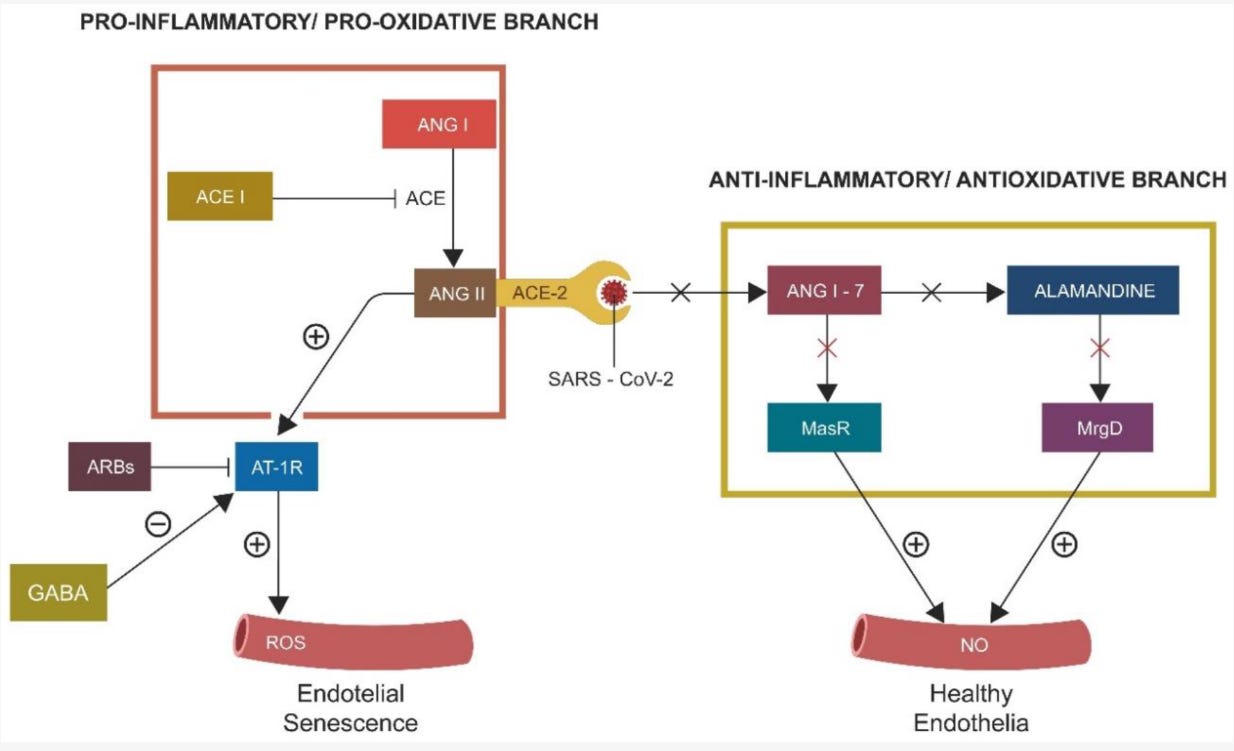
Figure 2. Human RAS consists of two opposing branches, the proinflammatory/prooxidative (driven by ANG II) branch and the anti-inflammatory/antioxidant (driven by ANG 1-7) one. ANG II, acting via AT-1Rs, induces EC senescence. ARBs and GABA negatively regulate AT-1Rs, opposing ANG II. The protective RAS branch, comprised of ANG 1-7, alamandine, and their respective receptors Mas and MrgD, inhibit inflammation and oxidative stress. SARS-CoV-2 engagement with ACE-2 disrupts the entire anti-inflammatory/antioxidant branch, leading to unchecked ANG II accumulation and premature EC senescence. Legend: ANG I, angiotensin I, ACEi, angiotensin-converting enzyme inhibitors, ANG II, angiotensin II, ARBs, angiotensin receptor blockers, AT-1r, angiotensin receptor type 1, ROS, reactive oxygen species, ANG1-7, angiotensin 1-7, MasR, Mas receptor, MrgD, MrgD receptor, NO, nitric oxide.
The viral spike (S) protein contains a GABA-mimicking sequence or short linear motif that can directly usurp host GABAergic signaling.
Yapici-Eser, H.; Koroglu, Y.E.; Oztop-Cakmak, O.; Keskin, O.; Gursoy, A.; Gursoy-Ozdemir, Y. Neuropsychiatric Symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions. Front. Hum. Neurosci. 2021, 15, 656313.
The reason this relates to SPED, is that:
Endothelial Cells (ECs) line the inner layer of the circulatory system and regulate the vascular function via membrane-bound receptors that interact with various neurotransmitters, hormones, and metabolites. While previously conceptualized as passive components of membranes and biological barriers, ECs are now known to play an essential role in vascular homeostasis and the pathogenesis of thrombosis and inflammation. Under normal circumstances, ECs synthesize and secrete eGABA, a molecule depleted in the virus-induced cellular senescence phenotype. Aside from viral infections, EC senescence and low eGABA were associated with PTSD, anxiety, depression, autism, schizophrenia, and epilepsy, suggesting that the viral manipulation of this neurotransmitter may initiate or exacerbate neuropsychiatric pathology. Moreover, as human ECs express abundant ACE-2, a positive regulator of eGABA, SARS-CoV-2 could disrupt the GABAergic signaling directly. Indeed, ACE-2 variants with depleted GABA were linked to major depressive disorder, schizophrenia, bipolar disorder, and epilepsy, emphasizing the importance of RAS/GABA crosstalk for central nervous system (CNS) homeostasis.
Additionally:
Recent studies have shown that ECs can form cellular networks and communicate via Ca2+ waves, suggesting that information processing may take place at the vascular level. Likewise, astrocytes form physiological syncytia, a finding consistent with the Ca2+ wave hypothesis of information processing. In addition, the dysfunctional eGABA association with altered cortical circuits and behavior likely highlights the role of ECs in cognition. Indeed, ECs communicate with and shadow neurons throughout the brain, likely participating in cognitive processes mediated by Ca2+. Moreover, as Ca2+ drives the rudimentary memory of plants and unicellular organisms, an ancient modality of non-neuronal information processing is emphasized. Along this line, the antidepressant action of ketamine, based, at least in part, on its impact on calcium/calmodulin-dependent protein kinase II (CaMKII), likely implicates Ca2+ in emotional intelligence and cognition. This is important, as virtual screening studies documented the existence of a CaMKII system in the S protein of SARS-CoV-2, linking this pathogen to affective disorders. Moreover, non-neuronal information processing was reported in skeletal muscle, heart, and fascia, indicating that neuronal cells do not hold the exclusive monopoly on cognitive processes. Along this line, the acquisition of donor personality characteristics following heart transplantation, documented by numerous studies, may reflect EC-mediated cognition. This is in line with the hemo-neural hypothesis that connects information processing to endothelial blood flow.
Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry
https://www.mdpi.com/2571-841X/5/2/22/htm
So, we have the Spike Protein interfering with GABA signaling at a vascular level (perhaps also at a CNS level). I believe this is another newly discovered consequence of SPED. Also, it very interestingly can add another level of explanation to the sudden cardiac arrests (SCA) and sudden cardiac deaths (SCD) we have been observing.
The priority therefore lies with studies investigating whether (different kind of) stress-induced changes in brain functioning and alterations in GABA and glutamate are indeed associated with changes in ECG markers of SCA risk. Consequently, if mental stress indeed reduces GABA concentrations in the amygdala and changes cardiac electrophysiology, future studies should investigate whether an altered SCA risk is associated with changes in GABA-ergic metabolism based on medication use or a particular genetic profile. Based on these findings, pharmacological treatment and development could then be advanced to reduce SCA risk (i.e., GABA-ergic pharmacotherapy of cardiac arrhythmias). In view of the addictive properties of most GABA-ergic drugs, we would also like to advocate more studies into the efficacy of behavioral therapy to prevent cardiac arrhythmias. This could involve learning a more adaptive stress response using functional Magnetic Resonance Imaging (fMRI) neurofeedback or cardiac physiology output-based training of bodily stress regulation (biofeedback).
Anxiety, Mental Stress, and Sudden Cardiac Arrest: Epidemiology, Possible Mechanisms and Future Research
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8850954/
This may be very similar to the mechanism that causes sudden death in epilepsy (SUDEP):
As it involves hyperexcitable neurons, a basic assumption links the pathogenesis of epilepsy and the generation of synchronized neuronal activity with an imbalance between inhibitory [g-aminobutyric acid (GABA)-mediated] and excitatory (glutamate-mediated) neurotransmission (the more favorable assumption).
Omega-3s may be beneficial:
Several experimental and clinical studies have clearly demonstrated that complementary medical therapies,41 especially omega-3 fatty acids, are often tried by neurologists to control seizures.42 The first randomized trial of omega-3 supplementation in patients with chronic epilepsy were encouraging, demonstrating a transient effect on seizure control that was not confirmed by other research group, but additional trials are required.
Sudden unexpected death in epilepsy: an important concern
https://www.sciencedirect.com/science/article/pii/S180759322201585X
On an immunological level, we also must consider the Spike Protein’s ability to induce GABA Receptor Autoantibodies due to its mimicry/interaction with GABA B:
As for its postsynaptic effects, SARS-CoV-2 proteins interact both directly with receptors of GABA (mainly GABA B) and glutamate (mainly NMDA and metabotropic glutamate receptors) leading to change in the membrane resting-state potential and action potential, in addition to secondary messenger systems that may indirectly affect membrane resting-state potential, in addition to intracellular protein functioning.
Neuropsychiatric Symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions
https://www.frontiersin.org/articles/10.3389/fnhum.2021.656313/full
Indeed, this has been documented:
Postvaccinal GABA‐B receptor antibody encephalitis after ChAdOx1 nCoV‐19 vaccination
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9537891/
Alas, this reduction in GABA may explain what happened to Celine Dion. Although via the Spike Protein’s interactions and not antibodies against glutamic acid decarboxylase.
Background: Patients with stiff-person syndrome (SPS) have circulating antibodies against glutamic acid decarboxylase, the rate-limiting enzyme responsible for the synthesis of gamma-aminobutyric acid (GABA). Although the patients' symptoms of stiffness and unexpected spasms can be explained on the basis of reduced or impaired inhibitory neurotransmitters, such as GABA, it is unclear whether the level of GABA in the brains of these patients is reduced and, if so, whether the reduction is due to anti-glutamic acid decarboxylase antibodies.
Results: No abnormalities were noted on brain magnetic resonance images. A prominent and significant decrease in GABA level was, however, observed in the sensorimotor cortex and a smaller decrease in the posterior occipital cortex but not in the cingulate cortex or pons.
Conclusions: The reduction of brain GABA in patients with SPS supports the clinical symptoms and indicates that the inhibitory GABAergic pathways are involved in the disease. Regardless of the responsible autoantigens, in SPS autoantibodies block the function of GABAergic neurons and interfere with the synthesis of GABA but do not cause structural changes in the brain.
Brain gamma-aminobutyric acid changes in stiff-person syndrome
https://pubmed.ncbi.nlm.nih.gov/15956168/



Within the first three seconds of listening to her announcement I suspected there was a connection to the shot most likely. Thank you for your summation on HOW SPED fits in to her diagnosis. As always you are proving yo be ahead of the curve on putting the pieces of the puzzle together. Thank you once again.
Does anyone know, did Celine take the Pfizer or J&J?
Many years ago I remember seeing stiff person syndrome on a list of lesser known mitochondrial myopathies. Although it seems the science is not settled.