THE SPIKE PROTEIN CAUSES TO THE EXACT SAME DAMAGE TO THE ENDOTHELIUM AS IF IT HAD BEEN IRRADIATED
An explanation for the observed increase in Cancers
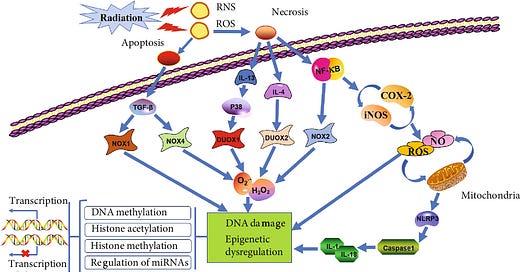
The damage caused by the spike protein mimics damage caused by radiation, damaging mitochondria and increasing NADPH oxidase resulting in persistent oxidative stress.
It is as though an atomic device has detonated in the bloodstream and fallout has descended all over the body. The actions of the Spike Protein PRECISELY mimic the actions of ionizing radiation within the body. It does this in the way heavy ion (LIKE IRON!) radiation does. This is done by damaging mitochondria and increasing NADPH production. This rise in persistent oxidative stress sets the stage for neoplastic transformation: oncogenesis.
This may be the most important finding from the Salk paper on the Spike Protein. They showed that the spike protein damaged the cells by binding ACE2. This binding disrupted ACE2’s molecular signaling to mitochondria (organelles that generate energy for cells), causing the mitochondria to become damaged and fragmented.
When you combine this with the S protein-induced selective activation of NADPH oxidase isoform 2 (NOX2) and reactive oxygen species (ROS) production that are ACE2-dependent, as well as ACE2 upregulation and induction of pro-inflammatory gene monocyte chemoattractant protein-1 (MCP-1) in endothelial cells to effectively attenuate endothelial dysfunction.
As stated in the headline, you are LITERALLY having your endothelium exposed to the EXACT SAME DAMAGE as if it had been IRRADIATED!
Delayed effects of radiation exposure have been attributed to persistent oxidative stress in tissues as a result of functional changes in mitochondria, a major source of reactive oxygen species (ROS) in cells. Although ROS are generated in mitochondria as a part of normal oxidative metabolism, enhanced ROS production could occur following damage to mitochondrial membrane and loss of mitochondrial membrane potential (MMP) as result of exposure to stressors like radiation and chemicals. Indeed, low-LET radiation exposure has been linked to mitochondrial membrane damage and alterations in MMP promoting ROS generation. NADPH oxidase, another important source of ROS, is activated by radiation exposure in murine hematopoietic stem cells leading to persistent oxidative stress and genomic instability.
What does this mean, ultimately? That long-term functional dysregulation of mitochondria and increased NADPH oxidase activity are major contributing factors towards heavy ion radiation-induced persistent oxidative stress in IEC with potential for neoplastic transformation (oncogenesis).
Referenced/Related Papers
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0042224