The Spike Protein and αIIbβ3: Understanding the Fibrous Clots from an Integrin-Mediated Perspective
The extensive clotting associated with the presence of the Spike Protein can be explained in the context of its affinity for all things connective tissue.
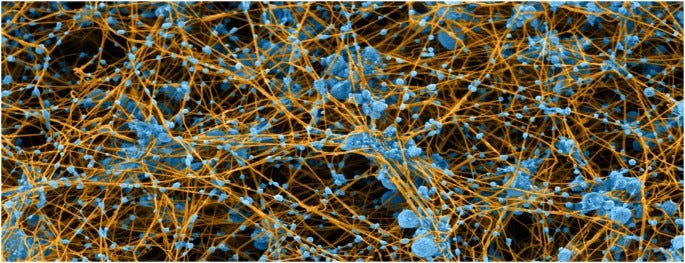
Colorized scanning electron micrograph of blood clot made by re-calcifying platelet-rich plasma. Platelets and microvesicles are colored blue and fibrin is tan. The micrograph illustrates the specific and intimate interactions between platelets and fibrin in blood clots and demonstrates that most fibrin fibers originate from platelet aggregates, where much of the thrombin is generated, while platelet-derived microvesicles decorate the fibrin fibers.
Like a Bond villain, the Spike Protein appears to use our connective tissue as its “hidden lair” from which it launches multiple attacks on the human body. We have been exploring how the Spike Protein binding to connective tissue proteins, such as those in the Extracellular Matrix (ECM) may induce cachexia and systemic organ damage. However, if we look very closely, we discover that these connective tissue proteins are also present in the blood. Indeed, it appears that the Spike Protein interacts with these blood-centric proteins in similar fashion.
Most people are not aware that the blood, like cartilage, is connective tissue! Integrins are proteins that, essentially, bind ECM and connective tissue components. Think Lego blocks!
Integrins are heterodimeric transmembrane receptors that mediate cell-adhesion.1 With their extracellular head region, most integrins bind extracellular matrix (ECM) glycoproteins such as laminins and collagens in basement membranes or connective tissue components like fibronectin. Others bind counterreceptors on neighboring cells, bacterial polysaccharides, or viral coat proteins. Through all these interactions integrins mediate stable adhesion to basement membranes, the formation of extracellular matrices and migration on such matrices, the formation of platelet aggregates, the establishment of intercellular junctions in the immune system, and bacterial and viral entry during infectious diseases.
Integrins: An Overview of Structural and Functional Aspects
https://www.ncbi.nlm.nih.gov/books/NBK6259/
The Bold emphasis above is mine. It is this mediation that can help us understand how the Spike Protein is able to induce such catastrophic clotting. Let’s look at one of these integrins – a platelet integrin. I know it may be difficult to conceptualize, but keep in mind the blood is connective tissue.
One of these “Lego block” proteins initiates clotting.
The formation of hemostatic plugs and obstructive thrombi are determined to a large extent by the integrin αIIbβ3-mediated interactions of platelets with fibrinogen and fibrin. The most studied of these interactions, fibrinogen binding to αIIbβ3, initiates platelet aggregation.
Strong Binding of Platelet Integrin αIIbβ3 to Fibrin Clots: Potential Target to Destabilize Thrombi
https://www.nature.com/articles/s41598-017-12615-w
So, the FORMATION of obstructive thrombi (clots) is DETERMINED by the INTEGRIN αIIbβ3.
And now to lift the curtain. The Spike binds this protein and potentiates platelet aggregation!
Platelet hyperreactivity is one of the crucial causes of coagulative disorders in patients with COVID-19. Few studies have indicated that integrin αIIbβ3 may be a potential target for spike protein binding to platelets. This study aims to investigate whether spike protein interacts with platelet integrin αIIbβ3 and upregulates outside-in signaling to potentiate platelet aggregation. In this study, we found that spike protein significantly potentiated platelet aggregation induced by different agonists and platelet spreading in vitro. Mechanism studies revealed that spike protein upregulated the outside-in signaling, such as increased thrombin-induced phosphorylation of β3, c-Src. Moreover, using tirofiban to inhibit spike protein binding to αIIbβ3 or using PP2 to block outside-in signaling, we found that the potentiating effect of spike protein on platelet aggregation was abolished. These results demonstrate that SARS-CoV-2 spike protein directly enhances platelet aggregation via integrin αIIbβ3 outside-in signaling, and suggest a potential target for platelet hyperreactivity in patients with COVID-19.
SARS-CoV-2 spike protein potentiates platelet aggregation via upregulating integrin αIIbβ3 outside-in signaling pathway
https://pubmed.ncbi.nlm.nih.gov/38981976/
Now we have a deeper understanding of how the Spike is able to use that which holds us together to tear us apart. Fortunately, there is some good news here.
Moreover, using tirofiban to inhibit spike protein binding to αIIbβ3 or using PP2 to block outside-in signaling, we found that the potentiating effect of spike protein on platelet aggregation was abolished.
I like that word in the context of the Spike Protein. Abolished. We will continue to learn, and we will continue towards abolishing the Spike Protein and its deleterious effects. And, yes, expect a Friday Hope on Tirofiban.
Thank you, as always, for your dialogue, readership and support. As I write in my Button text, I really cannot do this without you.


I’m wondering if regular use of low dose aspirin would benefit us since we’re constantly interacting with people who took the jabs given the shedding issue?
Using Colligen peptides and Vitamin K supplements can increase D Dimmer levels because of the effect that the spike has on Mast Cell Activation?