The Spike Protein, Acute Kidney Injury and Heart Failure: Induction of a Slow Death
The interplay between kidneys and the heart after Spike Protein injury may initiate a lethal feedback loop.
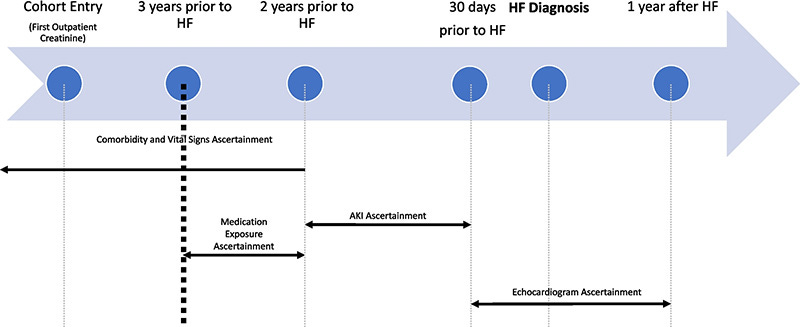
Ascertainment timeline. Time periods of ascertainment of baseline conditions, vital signs, medications, and exposure (AKI events) in relation to outcome (HF diagnosis date). Echocardiogram used to classify HF as HFpEF or HFrEF ascertained in 30 days before and up to 1 year following HF diagnosis date
One of the most glaring effects of the Spike Protein on the body (among many) is its apparent ability to induce Acute Kidney Injury (AKI). I believe this is something that clinicians need to be particularly aware of. After reviewing the evidence, it would seem prudent that any patients who have experienced AKI from COVID/Spike Transfection should be monitored for Heart Failure (HF). The connection between AKI and HF is the causative reason for concern.
First, let’s discuss how the Spike Protein may cause AKI. The most likely mechanism is the Spike Protein’s ability to mimic Acute Tubular Necrosis. It accomplishes this in a most devious manner.
The present study demonstrates that expression of the spike protein in HEK293-ACE2+ cells leads to cell fusion, progressive syncytia formation, and the lifting off and sloughing of sheets of fused cells from the monolayer, the latter giving rise to focal areas of denudation. These findings are reminiscent of what occurs during acute tubular injury/necrosis in AKI wherein injured renal epithelial cells, adhering to one another, detach from the tubular basement membrane and slough into the urinary space. The present study, to the best of our knowledge, is the first to call attention to this sloughing phenomenon in spike protein-expressing kidney cells and its mimicry of what may occur in the acute tubular necrosis variant of AKI. The relevance of syncytia to AKI is supported by a quite recent postmortem study in patients who died from COVID-19; in 28% of autopsy cases the presence of syncytia was reported in the kidney [23].
The spike protein of SARS-CoV-2 induces heme oxygenase-1: Pathophysiologic implications
https://pmc.ncbi.nlm.nih.gov/articles/PMC8669938/
It is not only SARS-CoV-2 Spike from infection that is significant. Spike transfection also appears to cause AKI.
Among the patients with post-vaccination AKI, the most common pathologic findings include crescentic glomerulonephritis (29.9%), acute tubular injury (23.7%), IgA nephropathy (18.6%), antineutrophil cytoplasmic autoantibody-associated vasculitis (17.5%), minimal change disease (17.5%) and thrombotic microangiopathy (10.3%).
New insights into kidney disease after COVID-19 infection and vaccination: histopathological and clinical findings
https://academic.oup.com/qjmed/article-abstract/117/5/317/7218935
The AKI is, of course, concerning in its own right as it may lead to conditions such as Chronic Kidney Disease. However, there is another connection to chronic disease that AKI has – and that is to Heart Failure (HF).
Preclinical data also provides insights on potential mechanisms of AKI-induced cardiac dysfunction, showing that AKI is associated with cardiomyocyte apoptosis, cardiac inflammation, mitochondrial dysfunction, and reduced ATP levels [21]. As both diastolic and systolic function are energy requiring processes, mitochondrial dysfunction leading to inadequate ATP levels is a plausible explanation of diastolic dysfunction in murine models (especially given the absence of traditional causes of diastolic dysfunction such as hypertension and severe acidosis) [8, 9]. A specific circulating mediator of AKI-induced cardiac dysfunction has not yet been identified, although proinflammatory cytokines and uremic toxins may play a role [20, 22]. Since AKI is known to induce systemic inflammation [23], which is a major driver of HFpEF pathogenesis [2], AKI-induced inflammation is a plausible potential mediator of the association with HFpEF.
In summary, we found that while AKI overall did not have a preferential association with HFrEF or HFpEF, stage 1 AKI had a statistically significant association with HFpEF. Our findings underscore the importance of close follow-up and attention to volume status and cardiac function after a hospitalization complicated by even mild AKI.
Heart failure subtype after acute kidney injury
https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-024-03602-1
It is because of this connection that I urge all clinicians who have patients that experienced AKI from either SARS-CoV-2 infection, Spike transfection, or both to monitor those patients for HF. Given the widespread knowledge that the Spike Protein almost certainly plays a role in inducing HF by directly affecting the heart itself. There may also be a feedback loop initiated as HF also contributes to CKD.
Fortunately, there also appear to be steps which can be taken to mediate AKI from the Spike Protein. More on this in this week’s Friday Hope. Thank you, as always, for your readership, dialogue and support.



So you just need to have contracted covid to have these symptoms because I wasn’t vaccinated, but I’m having issues similar to the one stated in this post thank you for posting
Or perhaps the administration of an organ toxic drug like Remdesivir is the issue.
- It was a failed ebola drug correct? It was withdrawn due to >50% death rate, correct?
- it attacked the organs, primarily the kidneys, correct?
Why is John Beaudoin incontrovertible proof of harms and intent being ignored by the freedom community?