The Spike as Autoimmune Pied Piper: After Invading the Endothelium, the Spike Induces Autoimmune Disease via an Integrin-Mediated Mechanical Process
The RGD sequence in the Spike Protein provides a comprehensive and convincing explanation for the Spike invading organs and inducing autoimmune disease.
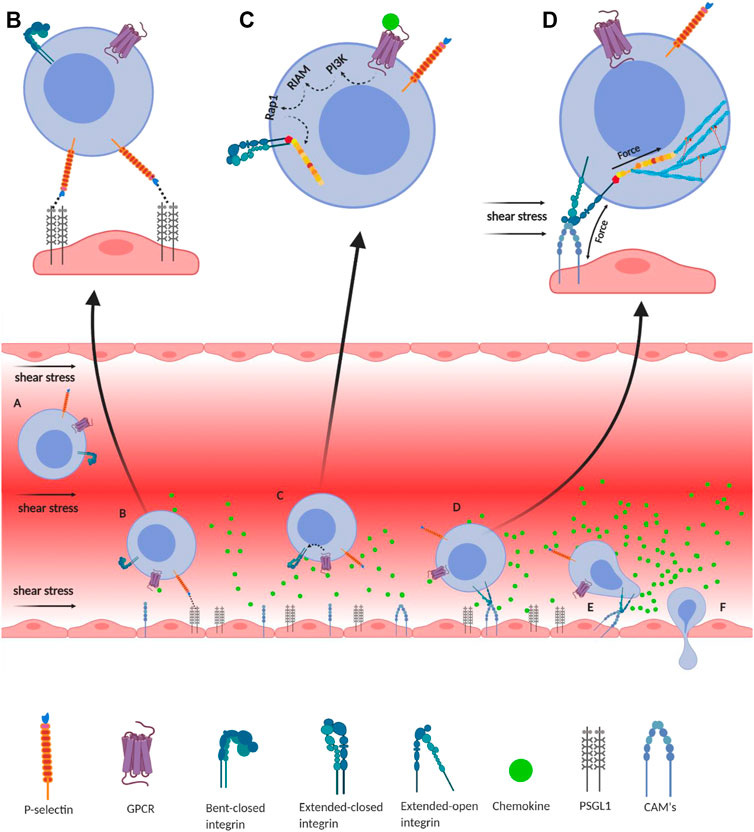
Integrin-mediated immune cell adhesion to endothelial cells under a shear force of blood flow. (A) Migration of immune cells under force—immune cells traveling through the blood vessel experience a shear force of the blood flow. Chemokines (green) are secreted by the endothelial cells lining the tissue displaying self-antigens; however, the chemokine gradient is highest near the infectious tissue. The chemokines slow down the flow rate of the migratory leukocytes towards the site of infection under the shear stress of blood flow, equivalent to 1 dyn/cm2. (B) Slip-bond formation and decrement in cell migration velocity—cells gradually decrease the speed along with the rise of chemokine gradient and tumble on the endothelial cells of the blood vessel. The selectin molecules, expressed by the leukocyte, interact with its counterpart expressed on the endothelial cells. However, their interaction under a shear force of blood flow causes the slippage of the bonds, allowing the cell to roll on the endothelial layer, while rolling numerous numbers of slip bond forms and breaks between the molecules like P-selectin, E-selectin, PSGL1, E-cadherin, etc. (C) Extended-closed integrins—the GPCR expressed on the leukocytes interacts with the chemokine to activate PI3K that induces Rap1–RIAM complex to activate talin for further binding with the β subunit cytosolic tail of integrin. This partially activates integrin from its bent-closed to extended-closed structure. (D) Integrin activation leading to focal adhesion—the extended-closed integrin gets activated, either by outside-in signaling by interacting with CAM while rolling on the endothelial layer or by inside-out signaling through sensing the force from talin–actin complex. The activation breaks the integrin salt bridge, transforming it into a thermodynamically unstable but active extended-open conformation. This forms integrin–ligand catch bonds under blood-flow shear force, resulting in complete adhesion of the immune cells to the endothelial layer. During this interaction, the force is transmitted through integrin both outside and inside the cell, which finally transduces downstream forming the focal adhesion. (E) Adhesion of cell—this focal adhesion regulates the cell's shape and migration and strictly adheres the cell on the endothelial layer by inducing the catch-bond formation. (F) Diapedesis—while remaining attached on the endothelial surface in the infected tissue, the self-reactive immune cells transmigrate in between adjacent cells by diapedesis towards the infected tissue region (Zhu et al., 2007b; Jahed et al., 2014; Huse, 2017).
With this post, I am finally able to tie together the appearance of SPED, Turbocancers and excess Autoimmune Disease. I propose that the Spike Protein’s invasion of the Endothelium, followed by its invasion of the Extracellular Matrix, explains all three. Thank you for supporting my work, it has allowed me to look very deeply and discover.
As readers of this Substack know, I have, from the beginning, called the Spike Protein’s invasion of the Endothelium Spike Protein Endothelial Disease (SPED). I then proposed that the Spike Protein subsequently traveled from the Endothelium into the body’s organs, where it would induce autoimmune disease and/or fibrosis. Yet, the actual mechanism of this progression has eluded me – until now.
What I have discovered is that the Spike Protein’s ability to bind Integrin provides a perfectly satisfactory explanation for this malevolent journey and its deleterious consequences. To start, let us look at some autoimmune diseases which are found to be Integrin Regulated Autoimmune Disorders.
Type 1 Diabetes Mellitus
Rheumatoid Arthritis
Multiple Sclerosis
Systemic Lupus Erythematosus
Type 1 Autoimmune Hepatitis
Scleroderma
Let’s look at some studies, shall we?
Vaccination is one of the most vigorous ways to intervene in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Cases of autoimmune hepatitis (AIH) after coronavirus disease (COVID-19) vaccination have been increasingly reported. Twenty-seven cases of AIH are summarized in this study, providing emerging evidence of autoimmune reactions in response to various COVID-19 vaccines, including in patients with special disease backgrounds such as primary sclerosing cholangitis (PSC), liver transplantation, and previous hepatitis C virus (HCV) treatment.
Autoimmune hepatitis after COVID-19 vaccination
https://pmc.ncbi.nlm.nih.gov/articles/PMC9732229/
The COVID-19 vaccination has been effective in preventing a lot of complications caused by SARS-CoV-2 and its variants. Meanwhile, diabetes mellitus, one of the root causes of many co-morbidities, exhibited itself during the COVID-19 pandemic and after COVID-19 vaccination. Diabetes mellitus introduced itself in a new perspective, leading to a variety of presentations and causing a significant number of emergency admissions. Many of the pre-diabetes patients with no prior history of diabetes developed fulminant type 1 diabetes mellitus (T1DM) after the COVID-19 vaccination. Some cases of conversion of type 2 diabetes mellitus (T2DM) into T1DM were reported. Some prediabetes/diabetes patients presented with the development of diabetic ketoacidosis after COVID-19 vaccination, whereas some previously healthy people with no relation to diabetes also developed acute exacerbations of new-onset T1DM or T2DM along with lethal ketoacidosis.
COVID-19 Vaccination and Its Relation to New-Onset Diabetes: A Narrative Review
https://pmc.ncbi.nlm.nih.gov/articles/PMC10644121/
Here, we report a patient diagnosed with SLE following Pfizer-BioNTech COVID-19 vaccination. This case highlights a potential risk of autoimmunity following vaccination with mRNA vaccines.
Systemic Lupus Erythematosus Following COVID-19 Vaccination
https://pmc.ncbi.nlm.nih.gov/articles/PMC10089130/
I believe you understand.
Now, let’s look at a principle of Integrin-Mediated Autoimmune Disease.
Only when these tolerance barriers are disrupted and self-reactive lymphocytes travel through the circulatory system to the site of inflammation or tissue displaying self-antigen does pathological autoimmunity develop (Xing and Hogquist, 2012).
Integrin Regulated Autoimmune Disorders: Understanding the Role of Mechanical Force in Autoimmunity
https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2022.852878/full
So, in essence, the Spike Protein becomes a lethal Pied Piper, leading the body’s own immune system directly into healthy tissue, attacking it and either by directly destroying it or inducing a fibrotic cascade, a long-term destruction.
In order to understand how this happens, it is first necessary to understand a unique property of the Spike Protein. In addition to many other nefarious attributes, it has one that gives it a rocket-booster ride into the ECM and the body’s organs. It is an RGD sequence!
Although ACE2 (angiotensin converting enzyme 2) is considered the primary receptor for CoV-2 cell entry, recent reports suggest that alternative pathways may contribute. This paper considers the hypothesis that viral binding to cell-surface integrins may contribute to the high infectivity and widespread extra-pulmonary impacts of the SARS-CoV-2 virus. This potential is suggested on the basis of the emergence of an RGD (arginine-glycine-aspartate) sequence in the receptor-binding domain of the spike protein. RGD is a motif commonly used by viruses to bind cell-surface integrins. Numerous signaling pathways are mediated by integrins and virion binding could lead to dysregulation of these pathways, with consequent tissue damage.
Biological and Clinical Consequences of Integrin Binding via a Rogue RGD Motif in the SARS CoV-2 Spike Protein
https://www.mdpi.com/1999-4915/13/2/146
We have the What and the Why. Now I will explain the How. This RGD motif allows the Spike Protein to become a very malevolent Pied Piper, leading the body’s own defenses on a self-attack!
The pathophysiology of autoimmune disorders is multifactorial, where immune cell migration, adhesion, and lymphocyte activation play crucial roles in its progression. These immune processes are majorly regulated by adhesion molecules at cell–extracellular matrix (ECM) and cell–cell junctions. Integrin, a transmembrane focal adhesion protein, plays an indispensable role in these immune cell mechanisms. Notably, integrin is regulated by mechanical force and exhibit bidirectional force transmission from both the ECM and cytosol, regulating the immune processes. Recently, integrin mechanosensitivity has been reported in different immune cell processes; however, the underlying mechanics of these integrin-mediated mechanical processes in autoimmunity still remains elusive. In this review, we have discussed how integrin-mediated mechanotransduction could be a linchpin factor in the causation and progression of autoimmune disorders.
The role of force-dependent integrin binding in cell–cell adhesion and cell–ECM interaction is indispensable. Different force-based imaging techniques have observed the biomechanics of leukocyte circulation, endothelial and trans-endothelial migration, and their persistence in the surrounding matrix (Schwartz et al., 2021).
These slip-bonds can be observed between E-selectin and their ligands, like different integrins, antibodies or antigens. (Li et al., 2016). Chen et al. (2011) showed that pulling force at a cyclic RGD motif bound to the integrin head also extended the integrin, suggesting force-dependent activation of integrins. The formation of catch bonds between integrins and their ligands is proved to be an important aspect of various immunological functions. For example, the LFA-1/ICAM1 interaction is majorly responsible for leukocyte migration and firm adhesion under force (Chen et al., 2010). Similarly, the fibronectin-receptor integrin α5β1 plays a direct role in angiogenesis (Kong et al., 2009). Integrin α4β1 (or VLA-4) is expressed on T and B lymphocytes, monocytes, eosinophils, neutrophils, and natural killer cells, promoting inflammatory responses by assisting leukocyte migration. Lastly, αMβ2 (or Mac-1) is another important integrin that is highly upregulated in migrating phagocytes (Lishko et al., 2003). These examples lead to the understanding that catch bond–slip bond transitions during the integrin–ligand interactions, under mechanical force-sensitive scenarios, will play crucial roles in immune cell mechanisms.
And that RGD sequence!
However, these degraded ECM peptides can act as major ligands in integrin activation, causing anomalies in mechanotransduction events. For example, we have discussed in the case of RA that MMP degrades collagen and that laminin frees the RGD peptide, which activates integrins, finally causing severe autoimmune disorder (Charo et al., 1990; Hoberg et al., 2006; Pankov and Yamada, 2002; Davis et al., 1990; Davis, 1992).
Integrin Regulated Autoimmune Disorders: Understanding the Role of Mechanical Force in Autoimmunity
https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2022.852878/full#B185
I have likened the Spike Protein’s path of destruction in the body to Sherman's March to the Sea. Now we know how that March happens.
It has been an honor to present this hypothesis to you. It ties together everything we have reviewed over the past five years and offers a thoroughly unifying hypothesis for Spike Protein injury and disease. I will continue to investigate the Spike’s invasion of our bodies, and how to counter it, and hopefully prevent/heal all the damage.
As always, thank you for your support, readership and dialogue. You make it all possible. Hope and blessings to everyone.



Best scientific explanation of how the bioweapon creates damage!
Thank you, Walter.