The Paper Published Yesterday by Biering, et al., Demonstrates That the Spike Protein Alone and Independent of ACE-2 Induces Spike Protein Endothelial Disease (SPED)
Lesson: How Spike Protein Exposure Could Lead to Systemic Sclerosis
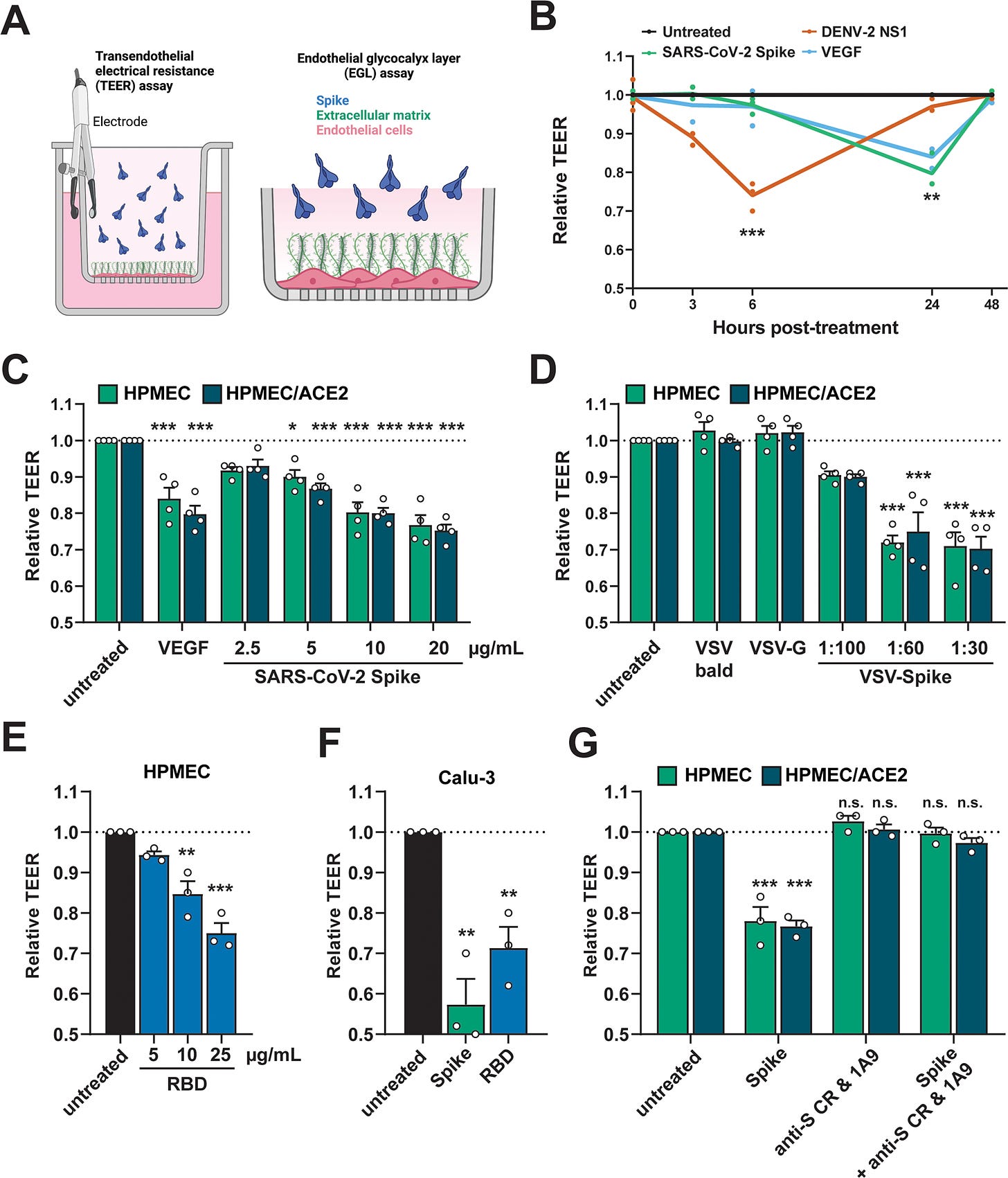
A Schematic depicting S-triggered barrier dysfunction measured by a trans-endothelial/epithelial electrical resistance assay (TEER; left) and an endo/epithelial glycocalyx layer (EGL) assay (right). B Time course of TEER assay measuring the barrier function of HPMEC monolayers over time with the indicated treatments, including DENV2 NS1 (5 µg/mL), VEGF (50 ng/mL), and SARS-CoV-2 S (10 µg/mL). Data are from n = 3 biological replicates. C A TEER assay measuring the barrier of monolayers of HPMEC and HPMEC/ACE2 at 24 hours after the indicated treatments. VEGF positive control (50 ng/mL). Data are from n = 4 biological replicates. D Same as C but treated with the indicated VSV-pseudotyped particles at the indicated dilutions. VSV-bald and VSV-G are diluted 1:30. Data are from n = 4 biological replicates. E Same as C but treated with the indicated concentrations of SARS-CoV-2 RBD. Data are from n = 3 biological replicates. F Same as C but measuring the barrier of Calu-3 cell monolayers. Data are from n = 3 biological replicates. G A TEER inhibition assay measuring the capacity of a cocktail of anti-S antibodies to inhibit S-mediated endothelial hyperpermeability. S (10 µg/mL) and the antibody cocktail (15 µg/mL of each antibody; 1A9 [Genetex] and CR3022 [Absolute Antibody]) were added simultaneously to the upper chamber of Transwell inserts to a monolayer of HPMEC or HPMEC/ACE2, and TEER was measured 24 hours post-treatment (hpt). Data are from n = 3 biological replicates. In all panels, the dotted line is the normalized TEER value of the untreated control condition. All data are plotted as mean + /− SEM. For all panels, values are compared to untreated controls by One-Way ANOVA with Tukey’s Multiple comparisons test except for (B), which was analyzed by two-sided unpaired t-test. *p < 0.05, **p < 0.01, ***p < 0.001, and n.s. p > 0.05. Source data are provided as a Source Data file.
A paper published yesterday confirms what I have hypothesized for years. The Spike Protein alone disrupts (damages) the (microvascular) endothelium and induces the overproduction of TGF-β. These are two primary components of what I have called Spike Protein Endothelial Disease (SPED) - damaged endothelium and subsequent fibrosis. Please see previous posts for more detailed explanation.
Our work indicates that levels of S observed in clinical samples from COVID-19 patients are sufficient to mediate barrier dysfunction (2.5 µg/ml). Our findings suggest that, in addition to functioning in viral entry, S interactions with GAGs and integrins induce vascular leak via activation of the TGF-β pathway. Further, our study offers a mechanistic explanation for the overproduction of TGF-β during COVID-19, which has been correlated with disease severity.
Also, they present the case for another aspect of SPED – how the Spike enters the bloodstream via the lungs to affect distal tissues. Isn’t this very similar to what cancers do?
It is important to note that our working hypothesis is not that S mediates disease pathogenesis alone, but rather that the reversible vascular leak triggered by S may serve to promote viral dissemination of SARS-CoV-2 into distal tissues of infected patients, which could lead to severe disease manifestations.
The authors also hypothesize what I have been claiming all along:
We also hypothesize that local concentrations of S accumulating in capillaries deep within tissues would likely be higher than levels circulating in patient sera. Thus, the concentrations of S we utilized in our study are consistent with circulating levels found in severe COVID-19 patients. However, the source of S that interacts with endothelial and epithelial cells to mediate barrier dysfunction during SARS-CoV-2 infection is still unclear.
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling
https://www.nature.com/articles/s41467-022-34910-5
Please note the text I bolded above. I believe the source of S may very well be S-encoding mRNA which persists post exposure.
Messenger RNA (mRNA) mediates the transfer of genetic information from the cell nucleus to ribosomes in the cytoplasm, where it serves as a template for protein synthesis. Once mRNAs enter the cytoplasm, they are translated, stored for later translation, or degraded. mRNAs that are initially translated may later be temporarily translationally repressed.
Messenger RNA regulation: to translate or to degrade
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2241649/
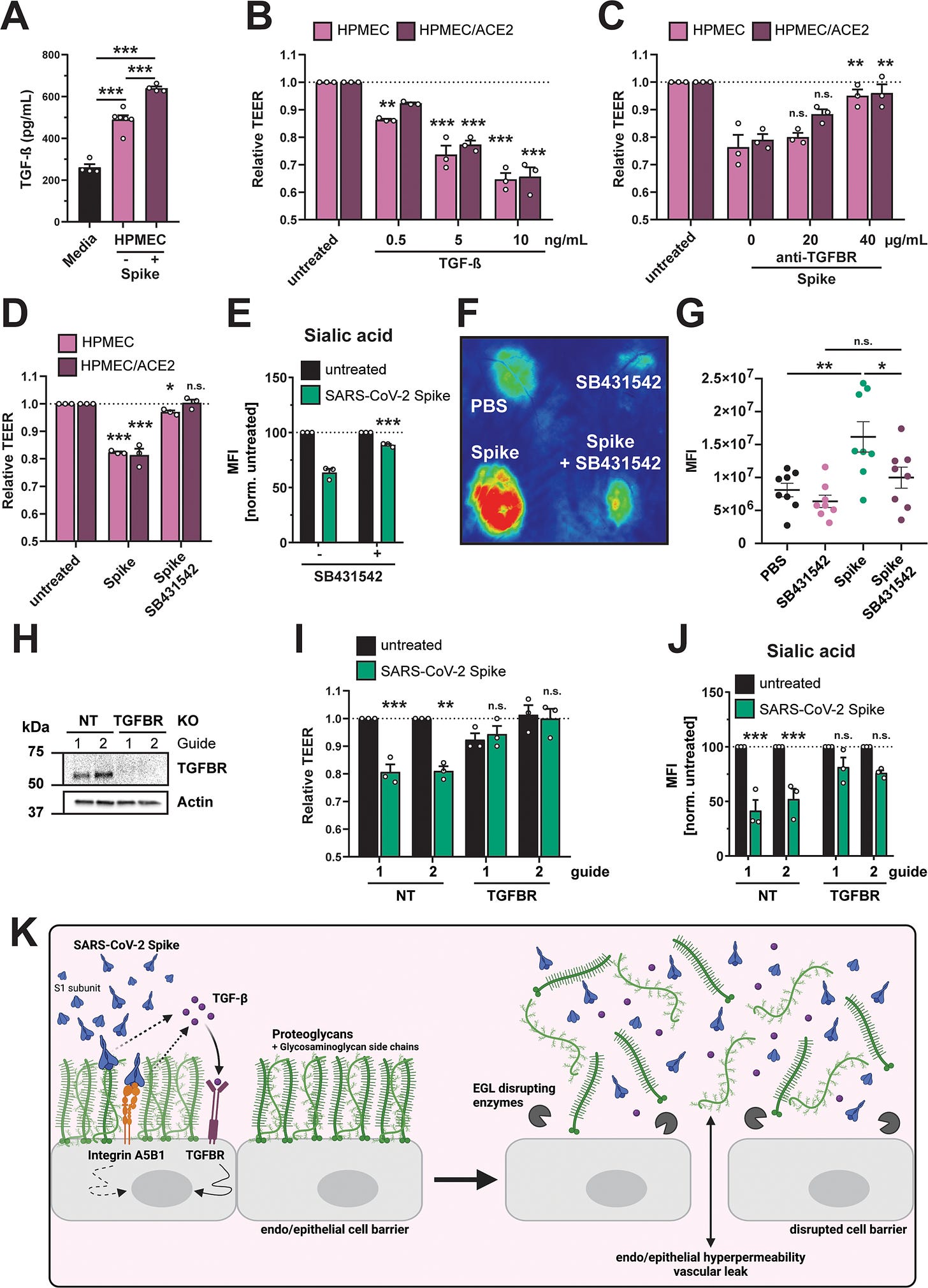
A Commercial ELISA detecting TGF-β in medium without cell conditioning (Media), medium from untreated HPMEC, and medium from HPMEC treated with 10 µg/mL SARS-CoV-2 S. Data are from n = 4 (media and HPMEC + S) and n = 6 (HPMEC) biological replicates. B TEER assay measuring the effect of recombinant TGF-β on barrier function of HPMEC at the indicated concentrations. TEER readings were taken 24 hpt. Data are from n = 3 biological replicates. C TEER assay measuring the capacity of an anti-TGFBR antibody, at the indicated concentrations, to abrogate S-mediated endothelial hyperpermeability (S at 10 µg/mL) of HPMECs and HPMEC/ACE2. TEER readings were taken 24 hpt. Data are from n = 3 biological replicates. D TEER assay measuring the capacity of TGFBR inhibitor SB431542 (1 µM) to inhibit S (10 µg/mL) function. Data are from n = 3 biological replicates. E Same as D, except an EGL assay measuring sialic acid. Data are from n = 3 biological replicates. F Representative back from an intradermal leak assay of mice with the indicated treatments; S (15 µg) and SB431542 (1 µM) were injected simultaneously. G Quantification of F from n = 8 mice. H Western blot analysis of HPMECs transduced with lentivirus-encoding guide RNAs targeting the indicated genes. Actin was used as a loading control. Data are one representative experiment from n = 3 biological replicates. I TEER assay on the same HPMEC as in H treated with 10 µg/mL of S and measured 24 hpt. Data are from n = 3 biological replicates. J EGL disruption assay on HPMECs from H, treated with 10 µg/mL S and imaged 24 hpt. Control guide data from this panel are from the same experiment as Fig. 4G. Data are from n = 3 biological replicates. K Graphical abstract summarizing the ACE2-independent pathway by which SARS-CoV-2 S triggers barrier dysfunction. Solid lines represent steps with direct experimental evidence, while dotted lines represent hypothesized steps. For all figures, dotted lines in graphs are the normalized untreated control conditions. MFI is mean fluorescence intensity. All data are plotted as mean + /− SEM with *p < 0.05, **p < 0.01, ***p < 0.001, and n.s. p > 0.05 by One-Way ANOVA with Tukey’s Multiple comparisons test except for (G) which was analyzed by two-sided unpaired t-test. Statistics in panels B, D, I, and J are comparisons to untreated controls and in panels C and E are comparisons to the S-only control condition. Source data are provided as a Source Data file.
THE DISCUSSION RAISES MORE QUESTIONS THAN IT ANSWERS
We will now address what I believe are the consequences of the upregulated TGF-β - Fibrosis and how the vascular leakage may induce Systemic Sclerosis.
First, let us look at the penultimate paragraph of the paper’s discussion.
However, although we observe significant vascular leak in mice administered S alone, they do not overtly display signs of morbidity. Importantly, our findings suggest that the amounts of S circulating in patients following COVID vaccination (pg/mL levels) are too low to trigger vascular leak given that our phenotype requires ng-µg/mL levels that mimic the levels observed during severe COVID-19 cases.
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling
https://www.nature.com/articles/s41467-022-34910-5
This is all disturbingly murky. How are the levels too low? What is the threshold? Isn’t it possible that the smallest vessels could be affected by a very small amount of circulating Spike? Also, one of the greatest dangers of SPED is that it may not overtly manifest.
The elevated TGF-β levels greatly concern me. Because TGF-β is the cornerstone of Fibrosis. One of the predicted outcomes of SPED.
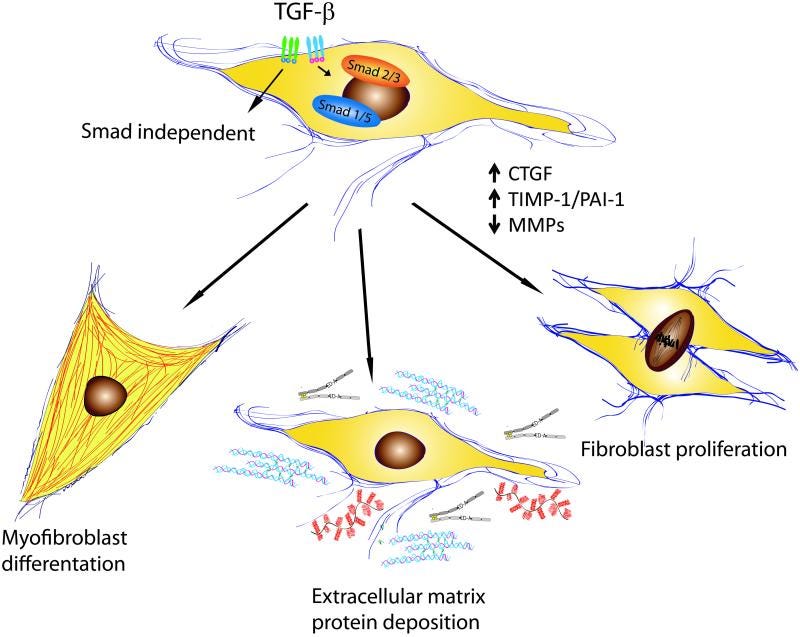
Effects of TGF-β on fibroblast phenotype and function.
Transforming growth factor β (TGF-β) is a central mediator of fibrogenesis. TGF-β is upregulated and activated in fibrotic diseases and modulates fibroblast phenotype and function, inducing myofibroblast transdifferentiation while promoting matrix preservation.
TGF-β signaling in fibrosis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4408550/
THE IMPLICATIONS FOR SYSTEMIC SCLEROSIS
Perhaps the greatest revelation of this paper is unintentional. It is now possible to have a clearer understanding of how SPED can induce Systemic Sclerosis. The vascular leaking induced by the Spike Protein is a textbook early pathogenic event. I offer the following paper for all to read.
The early phase of systemic sclerosis (SSc) presents edema as one of the main features: this is clinically evident in the digital swelling (puffy fingers) as well as in the edematous skin infiltration of the early active diffuse subset. Other organs could be affected by this same disease process, such as the lung (with the appearance of ground glass opacities) and the heart (with edematous changes on cardiac magnetic resonance imaging). The genesis of tissue edema is tightly linked to pathological changes in the endothelium: various reports demonstrated the effect of transforming growth factor β, vascular endothelial growth factor and hypoxia-reperfusion damage with reactive oxygen species generation in altering vascular permeability and extravasation, in particular in SSc.
Vascular Leaking, a Pivotal and Early Pathogenetic Event in Systemic Sclerosis: Should the Door Be Closed?
https://www.frontiersin.org/articles/10.3389/fimmu.2018.02045/full
We must understand exactly what the threshold is for circulating Spike to induce SPED. Is it related to vessel size? Is it related to multiple exposures? Does S mRNA become stored for later translation by cells?
We have so much more to learn. And we will. And we will find more therapeutics to deal with the damage and hopefully prevent it. Thank you, as always, for your support. I will continue to work.



God bless you Walter! Your tenacity and intellect is awe inspiring. Thank you for your work.
Might over-production of TGF-β also be related to the ‘turbo-cancer’ being observed?