The Great Disruptor: Spike Protein Endothelial Disease (SPED) is also Spike Protein EPITHELIAL Disease
How the Spike Protein invades intestinal epithelium and BBB allowing microbial/lipopolysaccharide translocation.
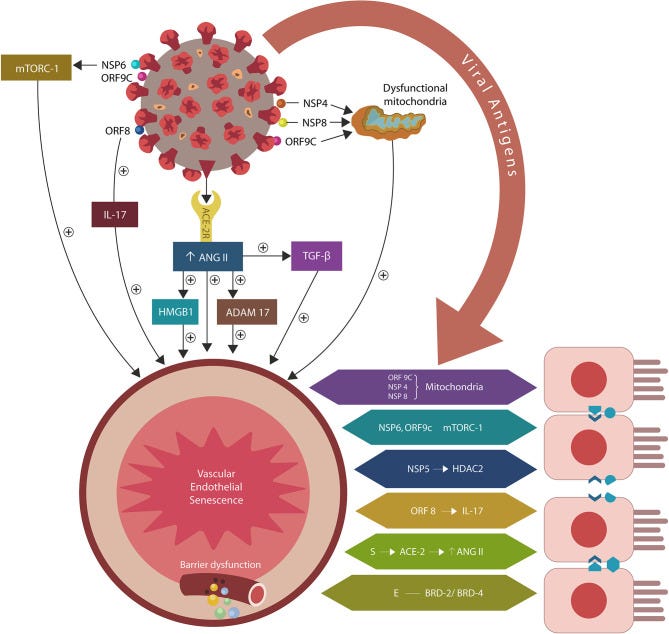
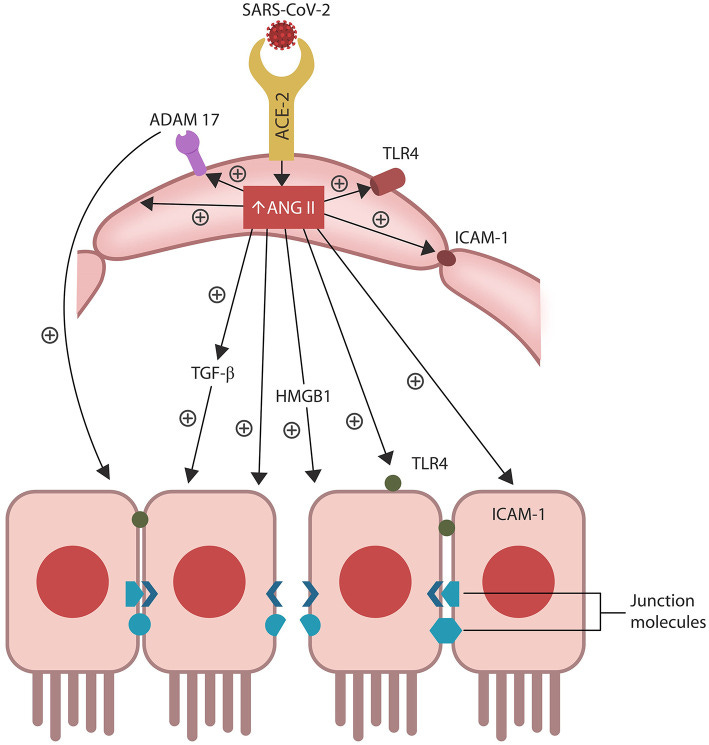
SARS-CoV-2/host protein-protein interactions. Viral crosstalk with several host proteins disrupts both endothelial cells (ECs) and intestinal epithelial cells (IECs), damaging the gut barrier and facilitating microbial and/or lipopolysaccharide (LPS) translocation into host tissues, including the skeletal muscle and the brain. Endothelial senescence also contributes to the disruption of blood-brain barrier (BBB), allowing microbial and/LPS access into the CNS. It is noteworthy that the cross talk between viral E antigen and host bromodomains (BRDs) 2 and 4 triggers macrophage senescence, impairing the elimination (efferocytosis) of aging cells. Viral S antigen attachment to ACE-2 receptor (ACE-2R) is followed by ANG II upregulation. This augments TGF-β, HMGB1, ADAM17, and ICAM-1 (not shown), inflicting endothelial and intestinal cells dysfunction with barrier disruption.
If you have been following my work for the past several years, you will almost certainly have read the paper I coauthored with Luc Montagnier on the ability of SARS-CoV-2 to induce senescence. We didn’t know all the details of HOW, yet, through observation, we hypothesized it was happening. For those who have not read the article, here is a link:
Le SARS-COV2 accélérerait l’âge biologique
https://www.francesoir.fr/opinions-tribunes/le-sars-cov2-accelererait-lage-biologique
One of the results of cellular senescence is that both the intestinal and blood brain barriers (BBB) experience increased permeability. And this is directly due to the actions of the Spike Protein.
ANG II accumulation in ECs alters several senescence-associated pathways, including ADAM17-downregulation of ACE-2 in ECs and IECs. ANG II-upregulated pathways include TGF-β, HMGB1, TLR4, ICAM-1, and SASP (not shown). Together these molecules induce cellular senescence, increasing intestinal and BBB permeability likely contributing to ME/CFS.
First, let us examine how the Spike Protein “teams up” with microbes in the gut to create a leaky gut and allow for microbial translocation – even to the CNS/brain.
According to this paradigm, the ME/CFS pathology is initiated by cellular senescence and barrier disruption promoted by the SARS-CoV-2-upregulated ANG II or by direct viral interaction with host proteins (Figure 1). When intestinal repair is delayed (due to specific host factors), the prolonged microbial translocation results in aberrant immune responses characteristic of both ME/CFS and COVID-19 critical illness (Loebel et al., 2016; Morris et al., 2019; Wang E. Y. et al., 2020; Wang L. et al., 2020).
The model presented here is supported by the SARS-CoV-2 interactome and the cross talk between microbial LPS and viral S protein that disrupt biological barriers (Gaab et al., 2005; Maes and Leunis, 2008; Giloteaux et al., 2016; Petruk et al., 2020). Indeed, recent studies in both humans and rodents linked LPS to the unexplained fatigue, myopathy, muscle wasting and memory impairment, likely implicating the endotoxin in the pathogenesis of ME/CFS (Langhans et al., 2014; Friedrich et al., 2015; Zhang et al., 2016; Batista et al., 2019; Lasselin et al., 2020). With the same token, LPS was recently connected to neurodegenerative disorders, indicating that this pathology may be initiated by the translocation of microbes and/or their molecules into the CNS (Pretorius et al., 2018; Zhan et al., 2018).
These aspects are presented below in more detail and in relation to the SARS-COV-2 virus, starting with the interactome and cellular senescence (both for ANG II-dependent and ANG II-independent molecular changes); ANG II and defective efferocytosis; and building the case toward the connection with gut biology. Lastly, the reviewed evidence is synthesized for potential therapeutic interventions, including senotherapeutic strategies.
Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8267916/
And, there is abundant evidence demonstrating how the Spike Protein induces senescence in epithelial cells as well as endothelial cells.
Increased mortality in COVID-19 cases is often associated with microvascular complications. We have recently shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein promotes an inflammatory cytokine interleukin 6 (IL-6)/IL-6R-induced trans signaling response and alarmin secretion. Virus-infected or spike-transfected human epithelial cells exhibited an increase in senescence, with a release of senescence-associated secretory phenotype (SASP)-related inflammatory molecules.
SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Cells
https://pubmed.ncbi.nlm.nih.gov/34160250/
One of the serious issues that these barrier permeability situations cause is that you have endotoxins flooding the body and even entering the brain. It is basically, like so many other pathological states in COVID, a microrepresentation of a much more dramatic state. In this case, it is a micro gut perforation.
A growing body of evidence indicates that the microbiome interacts with the central nervous system (CNS) and can regulate many of its functions. One mechanism for this interaction is at the level of the blood–brain barriers (BBBs). In this minireview, we examine the several ways the microbiome is known to interact with the CNS barriers. Bacteria can directly release factors into the systemic circulation or can translocate into blood. Once in the blood, the microbiome and its factors can alter peripheral immune cells to promote interactions with the BBB and ultimately with other elements of the neurovascular unit. Bacteria and their factors or cytokines and other immune-active substances released from peripheral sites under the influence of the microbiome can cross the BBB, alter BBB integrity, change BBB transport rates, or induce release of neuroimmune substances from the barrier cells. Metabolic products produced by the microbiome, such as short-chain fatty acids, can cross the BBB to affect brain function. Through these and other mechanisms, microbiome–BBB interactions can influence the course of diseases as illustrated by multiple sclerosis.
Gut reactions: How the blood–brain barrier connects the microbiome and the brain
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5788145/
One very interesting note: bacterial LPS can actually “hitch a ride” with the Spike Protein.
Moreover, as the S antigen of SARS-CoV-2 virus binds microbial LPS, and LPS-activated Tregs inhibit immune responses, the ME/CFS-associated endotoxin tolerance may be triggered via this mechanism (Lewkowicz et al., 2006; Morris et al., 2019; Table 2).
Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8267916/
Metformin appears to be quite helpful in ameliorating these barrier issues. I will be writing about that in an upcoming Friday Hope.
I am increasingly hopeful about much, and still very concerned about much. What still has me very concerned is that the Spike Protein appears to be causing pathologies that can be remedied, such as a leaky gut, and those which may not, such as inducing cancer or a prionopathy.
Thank you, as always, for your continued support, readership and dialog.


TYVM Walter for your ongoing work on our behalf.
A recent Mercola article provides actionable info on healing our microbiome and mitochondria:
https://takecontrol.substack.com/i/144267044/restoring-your-gut-microflora-and-cellular-energy-production-are-key
I didn't know you co-authored a paper with Luc Montagnier. That's awesome. This is great work you are doing.