Spike Protein Induced Heart Failure (HF): Significant Upregulation of IP10/CXCL10 Draws T Cells into the Heart
Infiltration of T Cells into heart tissue causes inflammation, fibrosis and cardiac remodeling resulting in heart failure.
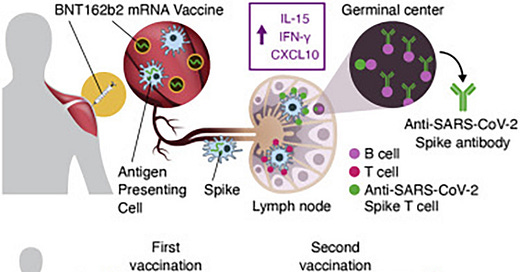
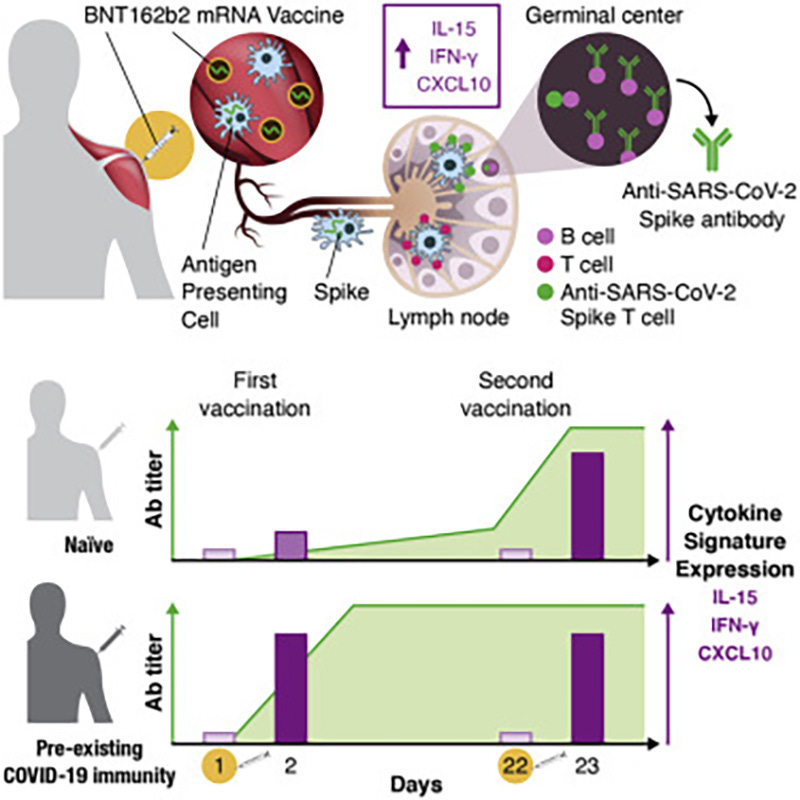
As evidenced by cytokine and chemokine responses to BNT162b2 mRNA, the chemokine IP10/CXCL10 is shown to be significantly overexpressed post injection.
The cytokine/chemokine response pattern was different at day 23 (1 day after the 2nd vaccination; Figures 2, S3, and 3D–3F). IFN-γ, IL-15, IP-10/CXCL10, and IL-6 showed elevated levels at day 23 that were significantly higher than those at day 2 (Figure 2). Remarkably, IFN-γ and IP-10/CXCL10 levels increased up to ∼20× and ∼4× over baseline after the 2nd vaccination, respectively. About 2× higher IL-15 and IL-6 peaks were detected after the 2nd vaccination (Figure 2).
Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients
https://www.cell.com/cell-reports/fulltext/S2211-1247(21)00932-3
Why is this significant? Because CXCL10 is a prime instigator of inflammatory processes which lead directly to HF. As evidence I present a paper from 2016.
Exciting new findings have now implicated adaptive immunity and T cells more directly as causal agents in hypertensive LV hypertrophy and resultant heart failure by poorly understood means. These preclinical studies employed the mouse model of transverse aortic constriction- (TAC-) induced heart failure to mimic the impact of high blood pressure on the heart. We observed that circulating levels of CXCL9 and CXCL10 are elevated in TAC mice [83]. Laroumanie et al. [84] reported increased recruitment of activated CD4+ and CD8+ T cells and elevated levels of several chemokines for T cells and monocytes, including CXCL10, in ventricular tissues from mice with TAC-induced heart failure. TAC-induced ventricular dilation and fibrosis was prevented and contractile dysfunction was attenuated in mice deficient in mature B and T lymphocytes due to knockout of RAG2, although cardiac hypertrophy was still observed. T cell replenishment in RAG2 knockout mice restored the TAC-induced heart failure phenotype. In addition, elimination of CD4+ T cells (MHCII knockout) but not CD8+ T cells (CD8+ knockout) prevented TAC-induced cardiac fibrosis and failure, suggesting a critical involvement of T helper cells. This conclusion was further supported by the observation that mice with transgenic T cell receptor specific for ovalbumin did not develop heart failure and fibrosis with TAC. Altogether these findings suggest that activation of CD4+ T cells in hypertension causes interstitial and perivascular fibrosis that leads to functional and morphological changes in the heart conducive to the development of heart failure. However, it should be noted that an earlier study reported that coronary vessels of RAG1 knockout mice exhibited more intimal hyperplasia and perivascular fibrosis compared to wild-type mice following TAC [85]. The basis for the discrepant findings of the two studies is not clear. More recently, Nevers et al. [86] also investigated the role of T cells in cardiac remodeling in response to TAC-induced pressure overload. They observed that the development of systolic dysfunction was associated with the kinetics of T cell infiltration into the left ventricle and evidence was provided that most of the infiltrating T cells were IFN-γ secreting Th1 cells. LV systolic and diastolic function were preserved with TAC in T cell deficient mice (T cell receptor (TCR) knockout), and LV hypertrophy, fibrosis, and inflammation were markedly attenuated. In addition, T cell depletion with an anti-CD3 antibody prevented heart failure in wild-type mice. Unresolved at present is the identity of the antigen(s) responsible for T cell activation in LV hypertrophy and heart failure, and the potential contribution played by the loss of regulatory mechanisms that normally protect the heart from T cells [87].
The CXCL10/CXCR3 Axis and Cardiac Inflammation: Implications for Immunotherapy to Treat Infectious and Noninfectious Diseases of the Heart
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5066021/
How can I explain this to the layperson? Easily.
Think of it this way. The Spike Protein throws a “bone” (CXCL10) into cardiac tissue (oh, yes, others too) and tells the immune system to “go fetch.” I think you now understand...
The result of the aberrant immune response, after sustained “fetching,” is to have a heart that is continually being attacked by the body and repaired. Attacked and repaired. This results in, essentially, scar tissue forming where heart tissue should be. Eventually, and predictably, the heart fails to do its job.
Interestingly, this can also explain why African Americans have fared worse when confronted with SARS-CoV-2 and its Spike Protein.
Biomarkers of chronic inflammation (such as C-reactive protein) have long been associated with cardiovascular disease and mortality; however, biomarkers involved in antiviral cytokine induction and adaptive immune system activation remain largely unexamined. We hypothesized the cytokine interferon gamma inducible protein 10 (IP-10) would be associated with clinical and subclinical cardiovascular disease and all-cause mortality in African Americans. We assessed these associations in the Jackson Heart Study (JHS) cohort and the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. There was a modest association of IP-10 with higher odds of left ventricular hypertrophy (OR = 1.20 (95% confidence interval (CI) 1.03, 1.41) per standard deviation (SD) higher natural log-transformed IP-10 in JHS). We did not observe associations with ankle brachial index, intima-media thickness, or arterial calcification. Each SD higher increment of ln-transformed IP-10 concentration was associated with incident heart failure (hazard ratio (HR) 1.26; 95% CI 1.11, 1.42, p = 4x10-4) in JHS, and with overall mortality in both JHS (HR 1.12 per SD, 95% CI 1.03, 1.21, p = 7.5x10-3) and REGARDS (HR 1.31 per SD, 95% CI 1.10, 1.55, p = 2.0 x 10−3), adjusting for cardiovascular risk factors and C-reactive protein. However, we found no association between IP-10 and stroke or coronary heart disease. These results suggest a role of IP-10 in heart failure and mortality risk independent of C-reactive protein. Further research is needed to investigate how the body’s response to chronic viral infection may mediate heart failure and overall mortality risk in African Americans.
Interferon gamma-induced protein 10 (IP-10) and cardiovascular disease in African Americans
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7117698/
I hope this post will generate interest in this mechanism and inspire others to join in searching for therapeutics.
As always, thank you for your support. And, as always, I cannot do this without your support.


My layperson mind says to fix the problem, you have to find a way to eliminate/heal the scar tissue, or effect a greater chance of healing without scarring. Are there enzymes supplements that can do so? Does proper heart care supplementation like coQ10/ubiquinol, omega3 oils help heal the heart without scarring in the first place? And I'll say it once again, vitamin C plays a role in this. Please contact Dr. Tom Levy and pose this data to him. He's a cardiologist and one of the key physicians with an advanced understanding of ascorbic acid and cardiac health.
Excellent stack WMC .. and I do like the bone and fetch analogy to inflame and repair process of scarring.. my input may be of interest to other laypeople seeking solutions to any fibrosis within the body…
All disease processes end in “Itis” or “Otis”
Itis are infections… Otis are inflammatory conditions that cause fibrosis (scarring)
Yes reduce inflammation to reduce scarring but you need to address scarring that’s already occurred.. this is done through using protease enzymes because fibrous tissue is actually proteins left after repair of damaged tissue… which is why nattokinase serrapeptase lumbrokinase and bromelain are helpful.. they eat up residual pockets of scar tissue without eating up muscle or healthy tissue….
Lastly there is a botanical that directly acts on the myocytes … particularly the cell membranes between each longitudinal section of each heart cell to reduce inflammation thereby dampening down scar tissue.. called motherwort (leonurus cardiaca) yes it has the Latin for heart in its Latin name.