SPIKE PROTEIN INDUCED CYTOKINES AND B-CELL DYSREGULATION: ARE B CELLS OVERPRODUCING MONOCLONAL ANTIBODIES CAUSING AMYLOIDOSIS, AUTOIMMUNITY AND THE REPORTED BLOOD HYPERVISCOSITY?
Looking further into B Cell pathology and the Spike Protein
After discovering the activation of PI3K by the Spike Protein which can cause autoreactive B Cells to survive and enter the periphery, I was not satisfied with the mechanism as being the primary cause of observed autoimmunity. T Cells generally prevent these types of B Cells from fully activating, though the mechanism may still occur if T Cells are dysregulated as well. This needs further investigation.
However, I kept researching B Cells as I now believe antibodies are at the heart of COVID, Long COVID and Spike Protein pathology. I read a very interesting case report from Italy.
Laboratory findings in severe coronavirus disease (COVID)-19 may include lymphopenia, elevated D-dimer, lactate dehydrogenase (LDH), C-reactive protein (CRP), and ferritin.1 Several quantitative and functional abnormalities in lymphocyte populations have been reported in patients with SARS-CoV-2 infection, with evidence of depletion of cytotoxic T-lymphocytes and natural killer cells. Lymphopenia has been identified as a prognostic marker for poor outcomes as it could be in correlation with cytokine storm. Though numerically depleted, circulating reactive lymphocytes were detectable in a consistent proportion of patients, some of them with a lymphoplasmacytic phenotype. Whether this cell population is related to polyclonal or monoclonal hypergammaglobulinemia is still unknown.
In conclusion, based on these data it could be hypothesized that the presence of a monoclonal spike during the inflammatory phase could reflect the degree of immune hyperactivation in patients with severe COVID-19. Further studies are needed to evaluate its frequency, long-term evolution and prognostic role in this clinical setting.
“Acute” monoclonal gammopathy in severe COVID-19
http://www.htct.com.br/en-acute-monoclonal-gammopathy-in-severe-articulo-S253113792030064X
What caused me to look further into this is the fact that monoclonal gammopathies cause the blood to become hyperviscous. I have read myriad reports of blood being virtually impossible to be drawn. Of blood being to “slow” and “thick” to be taken for labs. The presence of a monoclonal gammopathy would be an explanation.
Evaluation of hyperviscosity in monoclonal gammopathies
https://www.amjmed.com/article/0002-9343(85)90540-6/pdf
ETIOLOGY
The Spike Protein induces an environment ideal for gammopathies.
We observed that spike (S) protein potently induced inflammatory cytokines and chemokines, including IL-6, IL-1β, TNFα, CXCL1, CXCL2, and CCL2, but not IFNs in human and mouse macrophages.
SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway
https://elifesciences.org/articles/68563
It is as if there is a new condition, a blur between Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma.
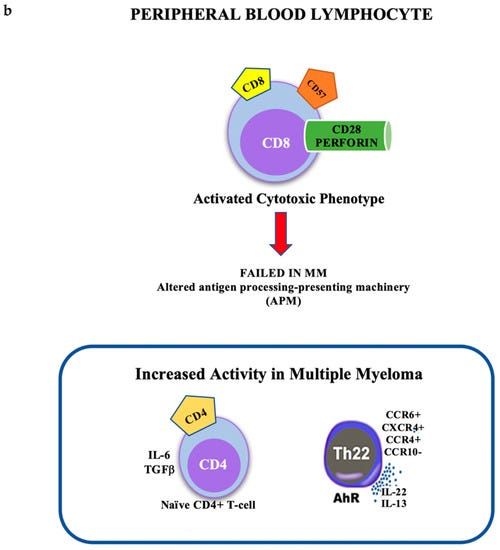
Lymphocyte Subsets and Inflammatory Cytokines of Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma
https://www.mdpi.com/1422-0067/20/11/2822/htm
AMYLOIDOSIS AND AUTOIMMUNITY
We return once again to aberrant proteins. This time, they are generated by plasma cells or monoclonal B-Cells, and they are called PARAPROTEINS.
The main focus of this article is immunoglobulin light chain amyloidosis (AL). In AL the fibrillary protein fragments are composed by misfolded clonal immunoglobulin-free light-chains produced and secreted by clonal plasma cells, or by a monoclonal B‑cell population. All premalignant entities associated with secretion of monoclonal paraprotein MGUS, SMM, CLL, FL, MCL, MALT-lymphoma and others, harbor the risk of paraprotein-triggered tissue damage. The mechanisms of paraprotein-driven tissue damage and systemic deterioration are multifarious and complex and a new and rapidly growing field of interest. A new group of rare and specialized paraprotein-initiated and driven diseases was established termed monoclonal gammopathy of clinical significance (MGCS). Based on the multiple mechanisms of tissue damage, varying categories of MGCSs are defined: organized depositions like fibrils or crystals versus nonorganized depositions; paraprotein-triggered autoimmune disorders; paraprotein-associated activation of alternative complement pathway; absorption of active molecules, or uncontrolled secretion of cytokines. The organs and systems involved within MGCS include kidney, peripheral nerves, skin, eyes, and hemostasis resulting in bleeding disorders. Based on this new knowledge the regular screening of MGUS patients should not be focused exclusively on progression to multiple myeloma but also on detection of paraprotein-associated organ damage.
Immunoglobulin light chain amyloidosis
https://link.springer.com/article/10.1007/s12254-021-00675-8
Please read the above referenced papers.




Thanks Walter. Great work again. I fear the end game fast approaches. MSM is allowing this to be viewed and read. Why? They want uproar, rebellion. They want martial law. Whatever happens now, thank you for all your hard work dedication. Hope to see people on the other side, whatever that looks like. Chaos is coming, take care, thanks
https://genervter-substack-com.translate.goog/p/ein-fass-ohne-boden-uber-toll-like?_x_tr_sl=de&_x_tr_tl=en&_x_tr_hl=de&_x_tr_pto=wapp
Oh dear Walter... It is so awful and there is a reason we are all discussing the same issues.
I would be honored if you would take the time to read my last part. I don't think you will need the crash course I mention at the beginning.
Best regards twittermitated 2Genervter (Annoyed Citizen) and now 3 Annoyed.