SARS-CoV-2 Spike Protein Binds to Heart’s Vascular Cells Potentially Contributing to Severe Microvascular Damage. A study from February of this year proves this, and explains the observed Myocarditis.
There is now extensive research regarding the instances of Myocarditis caused by both COVID-19 and the Spike Protein therapies. However, most people view this as a “side effect” of the therapies and a pathology of the virus. The truth of the matter is, I believe they are both the EXACT same mechanism.
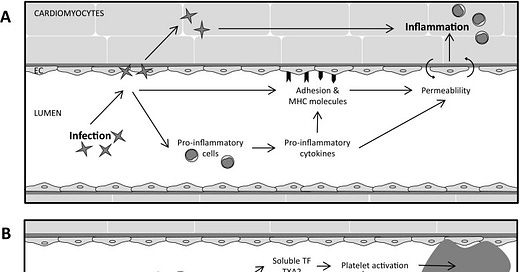
First of all, in addition to cardiomyocytes, endothelial cells of the cardiac (micro)vasculature are direct targets for infection. Myocarditis is an inflammatory disease of the heart that is characterized by a large diversity in symptoms varying from a SYMPTOMLESS course to shortness of breath and mild flu-like symptoms, chest pain, specific or a specific ECG changes, to acute heart failure and chronically to dilated cardiomyopathy.
In the heart, myocarditis can induce cell loss, interstitial and replacement fibrosis, wall motion abnormalities, decreased ejection fraction, and arrhythmias. Moreover, myocarditis is one of the leading causes of SUDDEN CARDIAC DEATH IN YOUNG ADULTS.
The cause of myocarditis can among others be an allergic or toxic reaction to medicines and toxic drugs as well as autoimmune organ-specific myocarditis and systemic autoimmune diseases-associated myocarditis. However, most often, the cause of myocarditis is an infection, including viruses, bacteria, protozoa, and fungi.
In our case, the cause is an TRANSFECTION with the Spike Protein of SARS-CoV-2. The infection of the cardiac endothelium can cause among others endothelial activation, damage, and permeability. For instance, infection of cardiac endothelial cells in patients with viral myocarditis has shown to induce endothelial microparticles reflecting endothelial damage.
We also observe the classic Spike/COVID-19 inflammation and thrombi as is seen in infectious myocarditis: The putative infection, pro-inflammatory activation, and death of cardiac endothelial cells each create a potential procoagulant environment. Indeed, occlusive thrombi, fibrin deposits, and aggregated platelets have been found in the small epicardial and intramyocardial vasculature of T. cruzi-infected mice and dogs and in mice with CVB3-induced myocarditis.
Clearly, the coronary (micro)vasculature plays a prominent role in the different stages of the disease; initially as a barrier against and as a target for infection, and subsequently as an important factor in the shaping of the immune response in the heart and as an important determinant of dysfunction of the heart. As such, these changes in the coronary (micro)vasculature may explain, in part, the wide variety of clinical symptoms in infectious myocarditis patients from COVID-19 and Spike Protein therapies.
Referenced/Related Papers
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6010496/
https://www.ahajournals.org/doi/full/10.1161/CIRCRESAHA.121.318902