Gultom, et al. Proving Spike Protein Endothelial Disease: Evidence for One of My Earliest SARS-CoV-2 Hypotheses
A preprint published this week provides conclusive evidence that SPED is real – and likely responsible for Long COVID.
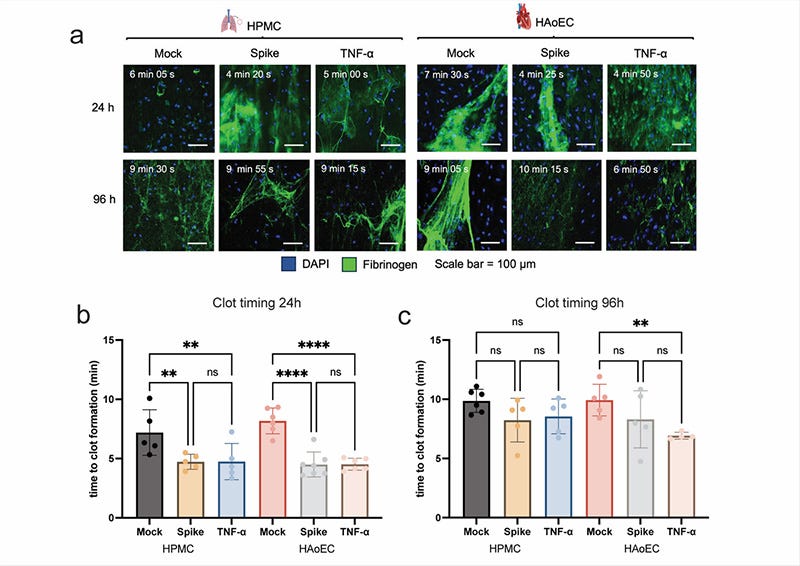
SARS-CoV-2 spike induces a procoagulant state of the human endothelium. SARS-CoV-2 spike and TNF-a-treated HPMC and HAoEC were perfused with recalcified citrated human plasma spiked with fluorescently labeled fibrinogen. Representative images of clot formation HPMC and HAoEC 24h and 96h post-treatment (a). Time to clot formation was determined from the time series imaging, defined as the time when complete occlusion of the channel and the formation of saturated fluorescence signal from the fluorescently labeled fibrinogen were observed. Clotting time for both ECs at 24h (b) and 96h (c) after treatment. One-way ANOVA with multiple comparisons was used for statistical analysis.
A preprint was published online this week that shows the Spike Protein of SARS-CoV-2 induces a diseased state of the endothelium, The constellation of findings precisely describes what I have called SPED (Spike Protein Endothelial Disease). It was four years ago that I hypothesized the Spike Protein would induce chronic inflammation of the endothelium with activation of coagulation cascades and high levels of cellular adhesion molecules.
The paper reads as a summary of my findings over the past four years:
In line with previous findings, we showed that spike-treated ECs express high levels of cellular adhesion markers. Increased ICAM1 expression on ECs mediates the recruitment and attachment of leucocytes and neutrophil extracellular trap (NET) formation, as well as a prothrombotic state of the endothelium. Indeed, we showed that treatment with SARS-CoV-2 spike directly triggered leukocyte adhesion and increased the procoagulant state of the ECs. Elevated ICAM1 in plasma, which could be released by the damaged endothelium, is also positively correlated with disease severity as has been observed in COVID-19 patients [51], [52]. Moreover, elevated ICAM1 and other EC adhesion molecules associated with disease severity have been described in chronic cardiovascular diseases including atherosclerosis and coronary heart disease [53], [54]. Our results also show that the expression of ICAM1 seems to persist beyond the presence of SARS-CoV-2 spike, suggesting a state of sustained inflammation of the ECs. Similarly, several studies have shown increased levels of ICAM1 in serum of patients recovered from COVID-19 [35], [55], [56]. The circulating ICAM1, which could originate from damaged endothelium, may contribute to prolonged inflammation even in recovered and no longer infectious COVID-19 patients, indicating the involvement of the endothelium in PASC.
Our results showed similar profiles of chemokine expression due to SARS-CoV-2 spike activation on human EC to those observed in COVID-19 patients [30], [31]. Several studies described that elevated IL-1β, IL-6, IL-8, IL-17 in plasma is associated with disease severity in COVID-19 patients [57], [58], [59], [60], [61]. Therefore, ECs may play a significant role in the production of various inflammatory cytokines and chemokines contributing to the cytokine storm and excessive inflammatory response, exacerbating the disease in severe COVID-19 patients [58], [62]. Expression of IL-1β, CXCL1, CXCL8, and CCL20 could contribute to neutrophil recruitment to the surface of the endothelium, leading to NET-formation and immunothrombosis [60], [61], [63], [64], [65]. Chemokines such as CCL8, CXCL2, and CXCL10 can lead to recruitment of monocytes and macrophages to the activated ECs [66], [67], [68]. The increased expression of CCL2 also contributes to the amplification of monocyte and macrophage activation [69]. Recruitment of immune cells to the surface of spike-activated EC can lead to infiltration of inflammatory cells and further damage to the surrounding tissue, which can happen independently of an active infection and in different anatomical regions. Different chemokine expression levels and dynamics over time between HPMC and HAoEC suggest a possible EC origin-specific response. Our RNAseq analysis further highlights the distinct transcriptomic signatures, and the pathways associated with SARS-CoV-2 spike activation of HPMC and HAoEC. It is therefore necessary to characterize organ-specific vascular responses from organs that are also affected by COVID-19, such as brain and kidneys.
Our RNAseq data further highlight that SARS-CoV-2 spike alone can trigger an array of pathogen-associated responses, induction of robust proinflammatory states, alteration of EC development, and apoptosis, likely associated with the observed thrombo-inflammatory symptoms in COVID-19 patients [70], [71]. Moreover, prolonged expression of genes associated with proinflammatory pathways and apoptosis could induce persistent endothelial dysfunction and damage. The observed prolonged increase of adhesion molecules and antigen presentation could lengthen the recruitment of immune cells and mediate EC interaction with CD8 + and CD4 + T lymphocytes [72], [73]. In addition, the transcriptomic analysis also showed a prolonged disruption of the regulation of the complement- and coagulation cascades, reflecting a possible sustained prothrombotic state and increased cardiovascular complications after COVID-19 infection. It is worth noting that we did not see significant changes in the leucocyte binding and clotting time at a later time point in vitro, which could be due to the limitation in the assay sensitivity in our model. It is, therefore, essential to validate the long-term changes due to SARS-CoV-2 spike activation in EC in a more extensive study, for instance, in animal models or clinical studies involving convalescent COVID-19 patients. In addition, future studies should consider evaluating the consequences of EC activation by SARS-CoV-2 beyond the indicated time points, as well as the vascular inflammatory effects of other SARS-COV-2 spike variants.
In summary, our results provided a detailed and comprehensive characterization of the vascular inflammatory effects of SARS-CoV-2. We showed that the endothelium plays an essential role in determining the outcome of COVID-19 infection, such as vascular inflammation and systemic organ damage during and possibly beyond the acute infection phase. Therapeutic strategies should also consider the extent of SARS-CoV-2 inflammatory effects on the vascular endothelium. Treatments directed to EC protection and prevention of endothelial damage might be essential in the prevention and management of the post-sequelae effect of COVID-19.
Sustained vascular inflammatory effects of SARS-CoV-2 spike protein on human endothelial cells
https://www.researchsquare.com/article/rs-5003230/v1
I take no joy in having been correct. However, I am thankful that, in having been correct, those who have read my work from the beginning have always known that protecting the endothelium from the Spike Protein has been paramount.

Thank you once again Walter, not the least bit surprised here. While I do not possess your level of expertise or scientific research credentials, I do have a strong suit in discernment. From very early on in this ongoing crime against the whole of humanity, you and a small handful of others led me to conclude that the real pathogenic culprit has been the Spike all along. SPED for sure. Sadly, I could not convince a sibling whose unquestioning faith in the med/pharma establishment complex has led to a precipitous decline in her health and quality of life across several bodily systems and functions. Even to this day she is unshakeable in her belief that "it would have been much worse if I had not ____________ (taken the jabs, etc., etc....)". What can one say? :( :o
There is theoretical knowledge and there is applied knowledge.
What about ending your article on a positive note where you translate theory into practice for those of us who do not have a medical degree? Could you add a nice little paragraph for non-medical people telling us how to "protect the endothelium from the Spike Protein". Any time you refer to this you can cut and paste the same paragraph to explain to any and every new non-medical reader how they can save their own lives. You could publish it as a note or an article in its own right, and just link to it whenever you raise the issue. That way your knowledge goes from theoretical to applied and will save lives.