Early-Life Exposome, Accelerated Epigenetic Aging and the Spike Protein
How repeated exposure to the Spike Protein may be rapidly advancing the biological age of youths.
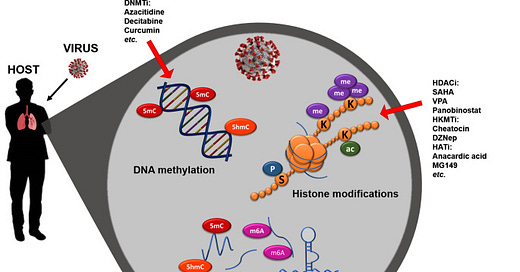
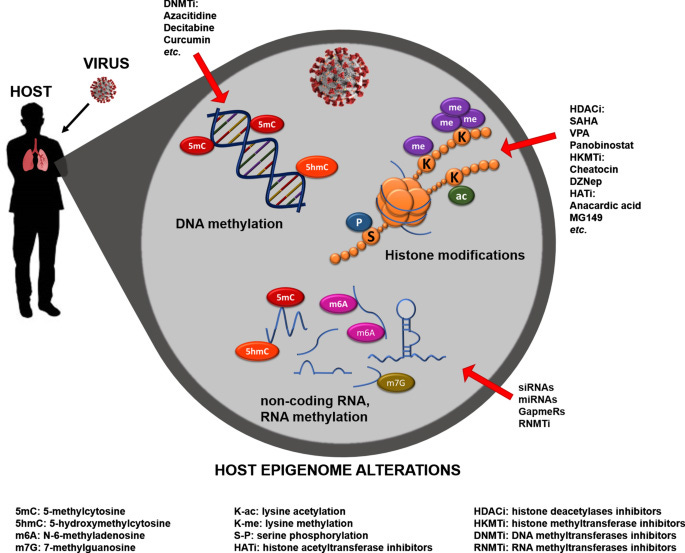
Coronavirus-dependent host epigenome alterations and potential interventions. Viruses, like those from the Coronaviridae family, can alter the host epigenome, negatively affecting the host immune response and successfully spreading the infection. The immune response is extensively regulated by specific epigenetic marks, such as chromatin remodeling, histone modification, DNA, and RNA methylation. The epigenetic machinery is responsible not only for the host response priming and memory, but also for ensuring its functional regulation. Age-related alterations to the host epigenome might affect the adaptive immune response, hindering viral defenses. Epigenomics represent a powerful tool to explore how to prevent, attenuate, or reverse the viral infection therapeutically. The enzymes responsible for the epigenetic alterations might represent potential targets for new antiviral drugs,
Though an entirely logical and seemingly obvious phenomenon, very few of us have considered that what we are exposed to in our youth can have serious health consequences later in life. Our biological “clocks” can be put into overdrive by what we are exposed to. Changes in gene expression, particularly those related to inflammation, can cause us to age much faster than we normally would. What we are exposed to in early life is known as the Exposome.
Early life represents a critical window of susceptibility to exposure to various environmental stressors collectively known as the early-life "exposome" (Miller, 2021 and Wright, 2017). Exposure during this all-important era of life can significantly influence the rate of aging as measured by DNA methylation-based epigenetic clocks (Horvath, 2013, McEwen et al., 2019, and Wu et al., 2019), which predict the age of an individual from DNA methylation levels at specific sites (Horvath and Raj, 2018). The early-life exposome significantly impacts human health and disease risk in later life (Barouki et al., 2012), with accelerated epigenetic aging proposed as an underlying mechanism. Much like biological age, increased epigenetic age has been linked to the onset of various age-related conditions, including cancer (Dugué et al., 2018), and overall mortality (Chen et al., 2016).
Overall, this fascinating study has highlighted how components of the early life exposome may negatively impact future health and increase disease risk in adult life by accelerating epigenetic aging and altering the epigenetic regulation of pathways involved in inflammation, detoxification, and cell cycle control. The authors propose that, considering aging as a worldwide public health issue, research such as theirs may help to reduce the exposure of children to environmental stressors and, in this manner, promote healthy aging from the early stages of life, which could reduce the burden on healthcare systems worldwide.
Early-life Exposome Accelerates Epigenetic Aging – Is There No Going Back Now?
https://www.activemotif.com/blog-exposome-accelerates-aging
The reason I am concerned is that pathways involved in inflammation are altered by the Spike Protein of SARS-CoV-2.
The researchers found that cultured human airway cells exposed to both low and high concentrations of purified S protein showed differences in gene expression that remained even after the cells recovered from the exposure. The top genes included ones related to inflammatory response.
Gene changes caused by Spike protein could explain long COVID
https://www.drugtargetreview.com/news/90224/gene-changes-caused-by-spike-protein-could-explain-long-covid/
It is not just in airway cells that the Spike Protein alters gene expression. It can also alter gene expression in neuronal cells.
Herein, we revealed that the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein acted as an extracellular remodeling component to undermine epigenetic regulation, perturb nascent RNA (nsRNA) transcription, promote mitochondrial biogenesis, and increase cellular viability in SH-SY5Y cells.
Epigenetic regulation of SARS-Cov-2 spike protein modulates cellular viability and nascent RNA transcription in neuronal cells
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10166610/
We need to perform vast testing on cells in all major organs. To what extent is the Spike Protein altering gene expression? In how many types of cells does this dysregulation remain after exposure?
Given all the research to date, the Spike Protein is almost certainly an age-shifter. This can explain why the young (initially) appear unaffected. Eight years on from seven, you are unlikely to be facing the diseases of old age at 15. The same is true for 22, 29, 35, etc. However, when you reach 65, 72 and older ages, jumping your biological clock ahead by seven years can, indeed, be fatal.
I will continue to research this. Fortunately, there are natural therapeutics which address gene regulation. I am working on a Friday Hope post discussing possible treatments. Thank you, as always, for your support, readership and dialogue.


And the insanity continues with wanting to get out saRNA.
Self Amplifying RNA shots. Anyone know what the true half life of even the modRNA is and the LNP once it is excreted in humans? In the study Iooked at in LNP process you can read that trillions of LNP'S are likely in each dose of these shots. Seems insanity to even consider that any sort of a preventative genetic therapy shot.
It's not nice to mess with mother nature.
.
Sudden Deaths
Of The Previously Healthy ?
That Takes A Violent Act.
These People Aren’t Dying Suddenly.
‘Not Even Close.
They Are Dying Violently.
.