BREAKTHROUGH: Does SARS-CoV-2 Infection/Spike Protein Exposure Initiate a Chronic Fibroblast Disease?
The Spike Protein activates fibroblasts, which can explain virtually every sequela of COVID/Spike Protein exposure.
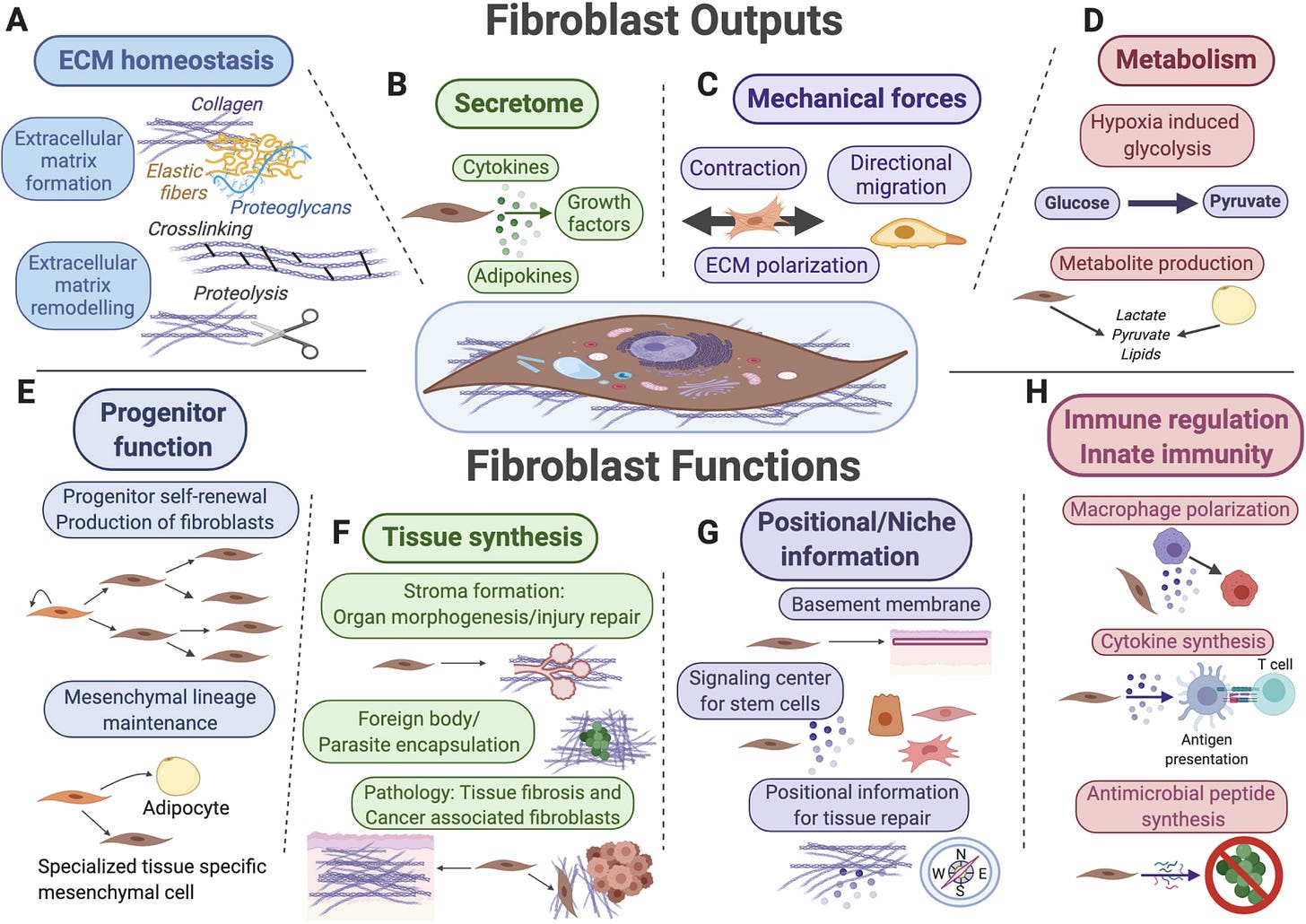
Key functions for fibroblasts (shown in the center) and their mesenchymal lineages include extracellular matrix (ECM) secretion and remodeling (A), secretion of signaling factors for surrounding cells (B), mechanical force generation (C), and regulation of tissue metabolism and metabolite secretion (D). Fibroblasts also function as progenitor cells for mesenchymal lineages (E), as “makers” of new tissue during organ morphogenesis, tissue repair and upon various pathological conditions (F), as sources of positional information across distinct anatomical regions of the same organ and as key signal contributors toward stem cell niches (G), as well as target cells and reciprocal modulators of diverse innate and adaptive immune functions (H).
Apologies for using BREAKTHROUGH for the second consecutive week, however, I believe the insight discussed today explains so much, that we need to, once again, reevaluate this “respiratory” disease.
As readers of this Substack know, last week I proposed that Spike Protein Endothelial Disease (SPED) was an initial stage of a disease which ultimately involves the Extracellular Matrix. I have been pursuing this over the past week and have found abundant evidence that this is almost certainly the case. In fact, it appears that the main actor in this ECM disease are the fibroblasts, which the Spike Protein activates.
Methods: We utilized scratch assays, Western blotting, and immunofluorescence to evaluate the migration, fibrosis signaling, mitochondrial calcium levels, reactive oxygen species (ROS) production, and cell morphology of cultured human cardiac fibroblasts (CFs) treated with spike (S1) protein for 24 h with or without an anti-ACE2 neutralizing antibody, a TLR4 blocker, or an NLRP3 inhibitor.
Results: S1 protein enhanced CFs migration and the expressions of collagen 1, α-smooth muscle actin, transforming growth factor β1 (TGF-β1), phosphorylated SMAD2/3, interleukin 1β (IL-1β), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). S1 protein increased ROS production but did not affect mitochondrial calcium content and cell morphology. Treatment with an anti-ACE2 neutralizing antibody attenuated the effects of S1 protein on collagen 1 and TGF-β1 expressions. Moreover, NLRP3 (MCC950) and NF-kB inhibitors, but not the TLR4 inhibitor TAK-242, prevented the S1 protein-enhanced CFs migration and overexpression of collagen 1, TGF-β1, and IL-1β.
Conclusion: S1 protein activates human CFs by priming NLRP3 inflammasomes through NF-κB signaling in an ACE2-dependent manner.
Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling
https://pubmed.ncbi.nlm.nih.gov/39195221/
If we examine SARS-CoV-2 sequelae, we may observe how activated fibroblasts may be the driving force behind them.
PULMONARY FIBROSIS
Many forms of pulmonary fibrosis involve the inexorable, progressive obliteration of parenchymal tissue which ultimately impairs gas exchange to cause respiratory failure and death. While no animal model accurately recapitulates all aspects of human lung fibrosis, bleomycin-induced lung injury can transiently increase the abundance of αSMA+ myofibroblasts and ECM in alveolar regions (Rock et al., 2011). Lineage tracing in this mouse model implicated numerous mesenchymal cells as the source for fibrotic myofibroblasts, including PDGFRα+ fibroblasts, lipofibroblasts, pericytes, and WT1+ mesothelial cells (El Agha et al., 2017; Hung et al., 2013). Such cooperative contribution of several tissue-resident mesenchymal cell types to fibrosis parallels analogous observations in other organs, including skin responses to wounding.
CARDIAC FIBROSIS/SUDDEN CARDIAC DEATH (ARRYTHMIAS)
Heart muscle is an anatomically and physiologically complex contractile organ, whose major cell population, cardiomyocytes connect with one another to form an electrically coupled tissue via intercalated disks that constitute its middle layer called myocardium. Heart is also rich in fibroblasts that generate and remodel a robust ECM network essential for electrical conductivity and heartbeat rhythm.
Fibroblasts: origins, definitions, and functions in health and disease
https://pmc.ncbi.nlm.nih.gov/articles/PMC8566693/#S12
DEMYELINATION/MS/NEUROLOGICAL SEQUELAE
Multiple sclerosis is an autoimmune disease. It has been reported that both PDGFRα+PDGFRβ+ meningeal fibroblast-like cells and PDGFRα−PDGFRβ+ pericytes increase and form a network in mouse meninges in the experimental autoimmune encephalitis model of multiple sclerosis.78 Upon inflammatory stimuli, such as TH17 (T-helper 17) cells and IL-17/IL-22, these cells secrete ECM proteins and cytokines, leading to enhanced immune response and demyelination.78 These findings suggest that meningeal fibroblast-like cells may play a detrimental role in experimental autoimmune encephalitis. Using Col1α1-GFP transgenic mice, it has been shown that perivascular fibroblast-like cells in the spinal cord become activated and infiltrate into neural tissue in experimental autoimmune encephalitis.79 Interestingly, this change is associated with animal behavioral deficits, demyelination, myeloid cell accumulation, and ECM deposition,79 indicating a detrimental role of perivascular fibroblast-like cells in experimental autoimmune encephalitis. In addition, fibroblast-conditioned media and fibroblast-derived ECM have been found to inhibit oligodendrocyte progenitor cell differentiation in vitro.79 Given that abnormal ECM deposition is observed in multiple sclerosis in both mice80 and humans,81,82 it is believed that fibroblast-like cells affect multiple sclerosis pathology and outcomes at least partially via their ECM proteins. This hypothesis and the underlying molecular mechanisms, however, need further investigation.
Central Nervous System Fibroblast-Like Cells in Stroke and Other Neurological Disorders
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.120.033431
TURBOCANCER EMERGENCE
Fibroblasts in the tumor stroma are well recognized as having an indispensable role in carcinogenesis, including in the initiation of epithelial tumor formation. The association between cancer cells and fibroblasts has been highlighted in several previous studies. Regulation factors released from cancer-associated fibroblasts (CAFs) into the tumor microenvironment have essential roles, including the support of tumor growth, angiogenesis, metastasis and therapy resistance. A mutual interaction between tumor-induced fibroblast activation, and fibroblast-induced tumor proliferation and metastasis occurs, thus CAFs act as tumor supporters. Previous studies have reported that by developing fibroblast-targeting drugs, it may be possible to interrupt the interaction between fibroblasts and the tumor, thus resulting in the suppression of tumor growth, and metastasis. The present review focused on the reciprocal feedback loop between fibroblasts and cancer cells, and evaluated the potential application of anti-CAF agents in the treatment of cancer.
Cancer associated fibroblasts: An essential role in the tumor microenvironment
https://pmc.ncbi.nlm.nih.gov/articles/PMC5588104/
INFLAMMATORY BOWEL DISEASE
Intestinal fibroblasts are pivotal players in maintaining tissue homeostasis and orchestrating responses to injury and inflammation within the gastrointestinal (GI) tract. Fibroblasts contribute significantly to the pathogenesis of inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis (UC), by secreting pro-inflammatory cytokines, modulating immune cell activity, and promoting fibrosis. In addition, fibroblasts play crucial roles in tissue repair and regeneration following acute injury or chronic inflammation. The dysregulation of fibroblast functions can lead to fibrotic complications, such as intestinal strictures and obstruction, which are common in advanced stages of IBD.
Fibroblast Heterogeneity in Inflammatory Bowel Disease
https://pubmed.ncbi.nlm.nih.gov/39684719/
LIVER/KIDNEY FIBROSIS
Activated fibroblasts are present in the injury response, tumorigenesis, fibrosis, and inflammation in a variety of tissues and myriad disease types. Summary: During normal tissue repair, quiescent fibroblasts transform into a proliferative and contractile phenotype termed myofibroblasts and are then lost as repair resolves to form a scar. When excessive levels are reached, activated fibroblasts proliferate and produce large amounts of extracellular matrix, which accumulates in the interstitial space of different organs. This accumulation leads to fibrotic dysfunction and multiple-organ dysfunction syndrome.
Metabolic Regulation of Fibroblast Activation and Proliferation during Organ Fibrosis
https://karger.com/kdd/article/8/2/115/824530/Metabolic-Regulation-of-Fibroblast-Activation-and
I will be researching therapeutics to ameliorate fibroblast activation, in addition to neutralizing the Spike Protein. My hope is that we may now be able to stem the adverse effects of the Spike Protein. This may ensure that we prevent the induction of cancers, neurological diseases and organ fibrosis.
I cannot thank you enough for your support, dialogue and readership. They are vital to my work as I am an independent researcher and not employed by any research or medical institution. If you are able to financially support my work, please consider doing so.



Thank you Walter. May God bless you and continue to guide you and your work. Peace.
Have we seen anything in medicine that causes as many problems as the 'spike protein?'