Asymptomatic SARS-CoV-2 Infection and Sudden Cardiac Death (SCD): Could Silent Infection/Reinfection with the SARS-CoV-2 Virus Be a Driving Force Behind the Observed SCDs?
A metabolic explanation mimicking “sudden morbid obesity.” The Spike Protein alone may also be a cause.
It is clear that there have been sudden cardiac deaths from the Spike Protein (SARS-CoV-2) due to Myocarditis. However, if we examine what occurs during an active SARS-CoV-2 infection, we discover that we are in a landscape ripe for SCD. It may be the case that those, especially athletes and those under great stress, suffering from cardiac arrest and SCD may be in the midst of an asymptomatic SARS-CoV-2 infection. This may be another significant mechanism for the observed dramatic increase in SCDs.
The key to this hypothesis is what occurs cellularly during a SARS-CoV-2 infection.
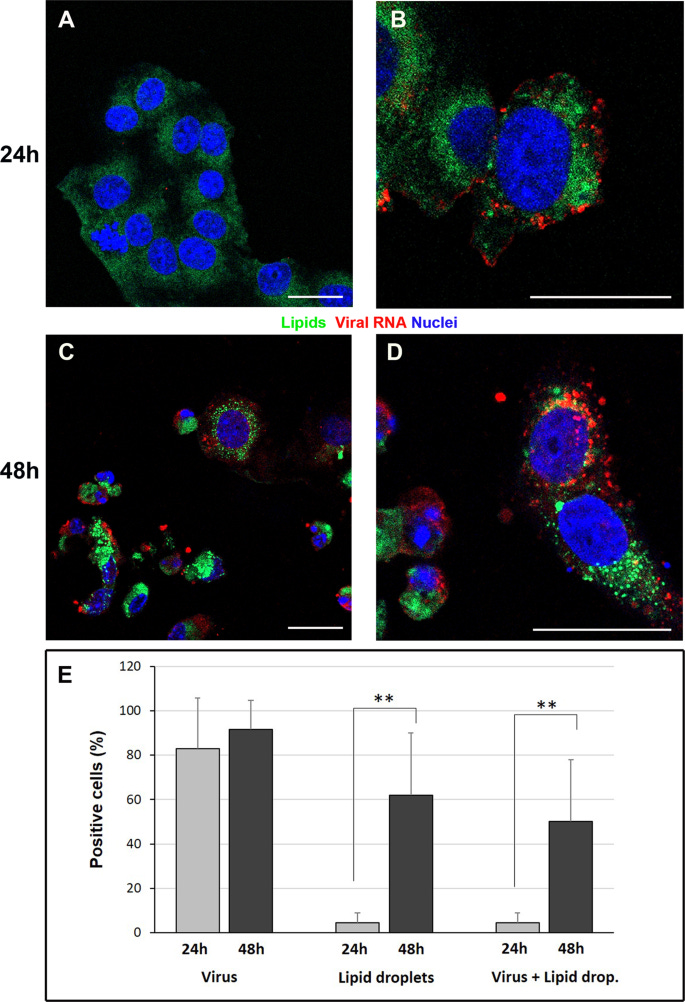
Immunofluorescence dual-labeling detection of viral dsRNA and lipid droplets. A Control cells, display absence of dsRNA signal and diffuse staining with lipophilic dye. B At 24 h post infection some cells showing viral RNA (red) also present lipid droplets (green dots) in the cytoplasm. C, D Numerous lipid droplets are visible in the cell cytoplasms (green dots) of cells 48 h post infection. E The graph shows the percentage of cells displaying viral RNA, lipid droplets staining or both labeling at 24 h and 48 h post infection. Data are mean ± SD from at least three independent experiments, and each experiment included duplicate samples. Statistically significant difference is showed (**p < 0.01). dsRNA (red); lipid droplets (green). Nuclei are stained blue (DAPI). Scale bars: A–D = 5 μm.
In conclusion, our findings demonstrate peculiar ultrastructural changes induced by SARS-CoV-2 infection. In particular, our work revealed that SARS-CoV-2 infection induce the accumulation of lipid droplets, both in cultured cells and in type II pneumocytes of lung from infected patients. These findings highlight a novel important open topic which may indicate new targets to contrast the pathogenicity of SARS-CoV-2. Several studies demonstrated that targeting host lipid metabolism by statins allows to suppress viral replication of many positive-strand RNA viruses, such as Hepatitis C virus, Dengueviruses, West Nile virus, and influenza A virus. Our results suggest that clinical studies, to assess the efficacy of statins on COVID-19 patient, or interfering with key lipid metabolic pathway enzymes could represent yet unconsidered therapeutic perspective.
Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis
https://www.nature.com/articles/s41419-021-03527-9
Indeed, the Spike Protein itself can affect lipid metabolism in the heart.
In contrast to controls, the spike protein was associated with impaired lipid metabolism and autophagic processes in the host cell. As a result, host cells were more readily affected by lipotoxicity, which was likely due to pathways triggered by the activation of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated ferroptosis.
In cell lines expressing the spike protein, there was a greater accumulation of lipids when compared to control cells, particularly on the cell membrane. In close association with this change, which signals impaired lipid metabolism, a number of enzymes and lipid-associated proteins were upregulated. Simultaneously, other markers of autophagy and ferroptosis also exhibited altered levels of expression.
SARS-CoV-2 spike protein disrupts lipid metabolism resulting in liver, heart & kidney damage
https://www.news-medical.net/news/20220421/SARS-CoV-2-spike-protein-disrupts-lipid-metabolism-resulting-in-liver-heart-kidney-damage.aspx
What is being missed, is that this is like throwing a metabolic switch and “instantly” transforming the victim into the cellular world of the morbidly obese – indeed a reason why the obese themselves tend to fare worse with the disease and viral protein.
What are the consequences?
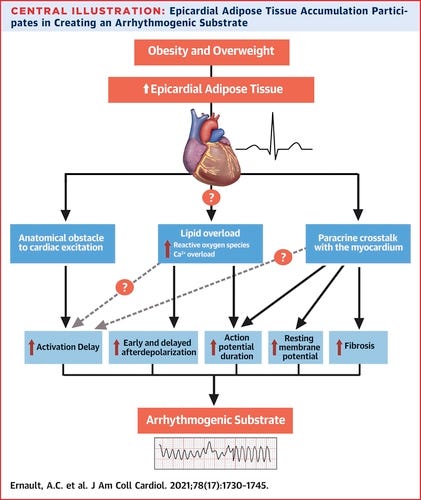
During Heart Failure, fatty acyl carnitines in the cytosol and sarcolemma are accumulated up to 10 times their normal levels, particularly in cardiac regions exposed to ischemia. The relationship between lipid accumulation and electrical abnormalities has been hypothesized in the 1980s (Corr et al., 1989). Since then multiple pathways have been established by which fatty acyl carnitines affect ion channel function and therefore arrhythmogenic events.
Changes in Myocardial Metabolism Preceding Sudden Cardiac Death
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7308560/
This would also explain why pilots, news anchors and others in stressful situations are succumbing to arrythmias causing cardiac arrest and sudden cardiac death. I urge all clinicians and research institutes to investigate this hypothesis immediately. Asymptomatic infection/exposure to the Spike Protein may be causing sudden death in ways less immediately obvious than fulminant or “mild” myocarditis.



Thank you Walter. May God bless you and guide you in your work. Peace.
Isn’t a more likely explanation the shot?